Ann Pediatr Endocrinol Metab.
2020 Dec;25(4):282-286. 10.6065/apem.2040076.038.
An A627V-activating mutation in the thyroid-stimulating hormone receptor gene in familial nonautoimmune hyperthyroidism
- Affiliations
-
- 1Department of Pediatrics, Pusan National University Hospital, Busan, Korea
- 23billion, Inc., Seoul, Korea
- 3Department of Laboratory Medicine, Pusan National University Yangsan Hospital, Yangsan, Korea
- 4Department of Pediatrics, Inje University Busan Paik Hospital, Inje University College of Medicine, Busan, Korea
- KMID: 2510901
- DOI: http://doi.org/10.6065/apem.2040076.038
Abstract
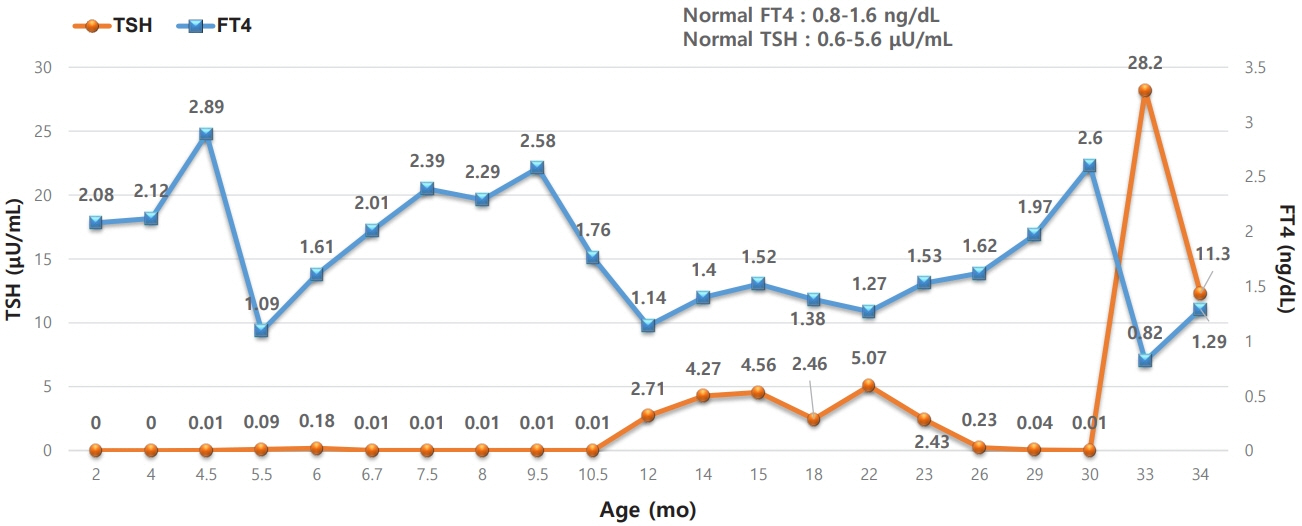
- Nonautoimmune hyperthyroidism is a very rare cause of congenital hyperthyroidism that is usually caused by an activating mutation in the thyroid-stimulating hormone receptor (TSHR) gene. In this report, we describe a case of nonautoimmune hyperthyroidism in a patient with TSHR mutation. Our patient was the younger of a set of twins born at 36 weeks and 6 days of gestation. The patient was noted to be more irritable than the older twin at 80 days of age, and the mother was taking methimazole for Graves’ disease that had been diagnosed 12 years prior. Therefore, a thyroid function test was conducted for the patient. The results revealed subclinical hyperthyroidism, and tests of antithyroglobulin antibody, antithyroid peroxidase antibody, and anti-thyroid-stimulating hormone (TSH) receptor antibody were all negative. During follow-up, at around 4 months of age, free T4 increased to 2.89 ng/dL, and TSH was still low at 0.01 μIU/mL; therefore, 3 mg/day of methimazole was initiated. Whole-exome sequencing showed a heterozygous variant of c.1800C>T (p.Ala627Val) in the TSHR gene. Testing in the family confirmed an identical variant in the patient's mother, leading to diagnosis of familial nonautoimmune hyperthyroidism inherited in an autosomal dominant pattern. This is the second report of A627V confirmed as a germline variant.
Keyword
Figure
Reference
-
References
1. Polak M. Hyperthyroidism in early infancy: pathogenesis, clinical features and diagnosis with a focus on neonatal hyperthyroidism. Thyroid. 1998; 8:1171–7.
Article2. Fuhrer D, Warner J, Sequeira M, Paschke R, Gregory J, Ludgate M. Novel TSHR germline mutation (Met463Val) masquerading as Graves' disease in a large Welsh kindred with hyperthyroidism. Thyroid. 2000; 10:1035–41.
Article3. Cho WK, Ahn MB, Jang W, Chae H, Kim M, Suh BK. Nonautoimmune congenital hyperthyroidism due to p.Asp633Glu mutation in the TSHR gene. Ann Pediatr Endocrinol Metab. 2018; 23:235–9.
Article4. Ferraz C, Paschke R. Inheritable and sporadic non-autoimmune hyperthyroidism. Best Pract Res Clin Endocrinol Metab. 2017; 31:265–75.
Article5. Seo GH, Park JY, Kim S, Lee J, Oh A, Lee Y, et al. High diagnostic yield and clinical utility of WES for patients with undiagnosed genetic disorder by automating variant interpretation. bioRxiv. 2019; 628438.6. Genome Aggregation Database (gnomAD) [internet] [cited 2019 Sep 14] Available from: https://gnomad.broadinstitute.org/.7. Ioannidis NM, Rothstein JH, Pejaver V, Middha S, McDonnell SK, Baheti S, et al. REVEL: an ensemble method for predicting the pathogenicity of rare missense variants. Am J Hum Genet. 2016; 99:877–85.
Article8. Clinvar-NCBI [internet]. Bethesda (MD): National Center for Biotechnology Information;[cited 2019 Sep 14]. Available from: https://www.ncbi.nlm.nih.gov/clinvar/.9. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015; 17:405–24.
Article10. Kim JH, Lee SS, Lim JS, Shin CH, Yang SW. Hyperthyroidism caused by a mutation in the thyrotropin receptor gene in two brothers. Korean J Pediatr. 2005; 48:337.11. Gozu HI, Bircan R, Krohn K, Muller S, Vural S, Gezen C, et al. Similar prevalence of somatic TSH receptor and Gsalpha mutations in toxic thyroid nodules in geographical regions with different iodine supply in Turkey. Eur J Endocrinol. 2006; 155:535–45.
Article12. Hebrant A, van Staveren WC, Maenhaut C, Dumont JE, Leclere J. Genetic hyperthyroidism: hyperthyroidism due to activating TSHR mutations. Eur J Endocrinol. 2011; 164:1–9.
Article13. Lueblinghoff J, Eszlinger M, Jaeschke H, Mueller S, Bircan R, Gozu H, et al. Shared sporadic and somatic thyrotropin receptor mutations display more active in vitro activities than familial thyrotropin receptor mutations. Thyroid. 2011; 21:221–9.
Article14. Matsuura N, Konishi J, Fujieda K, Kasagi K, Iida Y, Hagisawa M, et al. TSH-receptor antibodies in mothers with Graves' disease and outcome in their offspring. Lancet. 1988; 1:14–7.
Article15. Tamaki H, Amino N, Takeoka K, Iwatani Y, Tachi J, Kimura M, et al. Prediction of later development of thyrotoxicosis or central hypothyroidism from the cord serum thyroid-stimulating hormone level in neonates born to mothers with Graves disease. J Pediatr. 1989; 115:318–21.
Article16. Thomas JL, Leclere J, Hartemann P, Duheille J, Orgiazzi J, Petersen M, et al. Familial hyperthyroidism without evidence of autoimmunity. Acta Endocrinologica. 1982; 100:512–8.
Article17. Duprez L, Parma J, Van Sande J, Allgeier A, Leclere J, Schvartz C, et al. Germline mutations in the thyrotropin receptor gene cause non-autoimmune autosomal dominant hyperthyroidism. Nat Genet. 1994; 7:396–401.
Article18. Karges B, Krause G, Homoki J, Debatin KM, de Roux N, Karges W. TSH receptor mutation V509A causes familial hyperthyroidism by release of interhelical constraints between transmembrane helices TMH3 and TMH5. J Endocrinol. 2005; 186:377–85.
Article19. Fuhrer D, Wonerow P, Willgerodt H, Paschke R. Identification of a new thyrotropin receptor germline mutation (Leu629Phe) in a family with neonatal onset of autosomal dominant non-autoimmune hyperthyroidism. J Clin Endocrinol Metab. 1997; 82:4234–8.
Article20. Rivkees SA, Sklar C, Freemark M. Clinical review 99: the management of Graves' disease in children, with special emphasis on radioiodine treatment. J Clin Endocrinol Metab. 1998; 83:3767–76.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Nonautoimmune congenital hyperthyroidism due to p.Asp633Glu mutation in the TSHR gene
- A Case of Resistance Syndrome to Thyroid Hormone Associated with Mutation (G345D) in the Thyroid Hormone Receptor Beta Gene
- Hyperthyroidism Caused by a Mutation in the Thyrotropin Receptor Gene in Two Brothers
- Resistance to thyroid hormone due to a novel mutation of thyroid hormone receptor beta gene
- Monogenic Thyroid Disorder