Cancer Res Treat.
2021 Jan;53(1):199-206. 10.4143/crt.2020.497.
Prognostic Value of Serum Soluble Programmed Death-Ligand 1 and Dynamics During Chemotherapy in Advanced Gastric Cancer Patients
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea
- 2Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea
- KMID: 2510661
- DOI: http://doi.org/10.4143/crt.2020.497
Abstract
- Purpose
The soluble form programmed death-ligand 1 (sPDL1) has immunosuppressive properties and is being studied as a candidate biomarker for immuno-oncology drug development. We measured the serum sPDL1 at pre-and post-chemotherapy and evaluated its prognostic implication and dynamics during chemotherapy in advanced gastric cancer (GC).
Materials and Methods
We prospectively enrolled 68 GC patients who were candidates for palliative standard first-line chemotherapy, and serially collected blood at baseline and after one cycle of chemotherapy, at the best response and after disease progression. sPDL1 was measured using an enzyme-linked immunosorbent assay. Response to chemotherapy, overall survival (OS), progressionfree survival (PFS) and other prognostic factors including neutrophil-lymphocyte ratio (NLR) were obtained. The cut-off value of sPDL1 levels for survival analysis was found using C-statistics.
Results
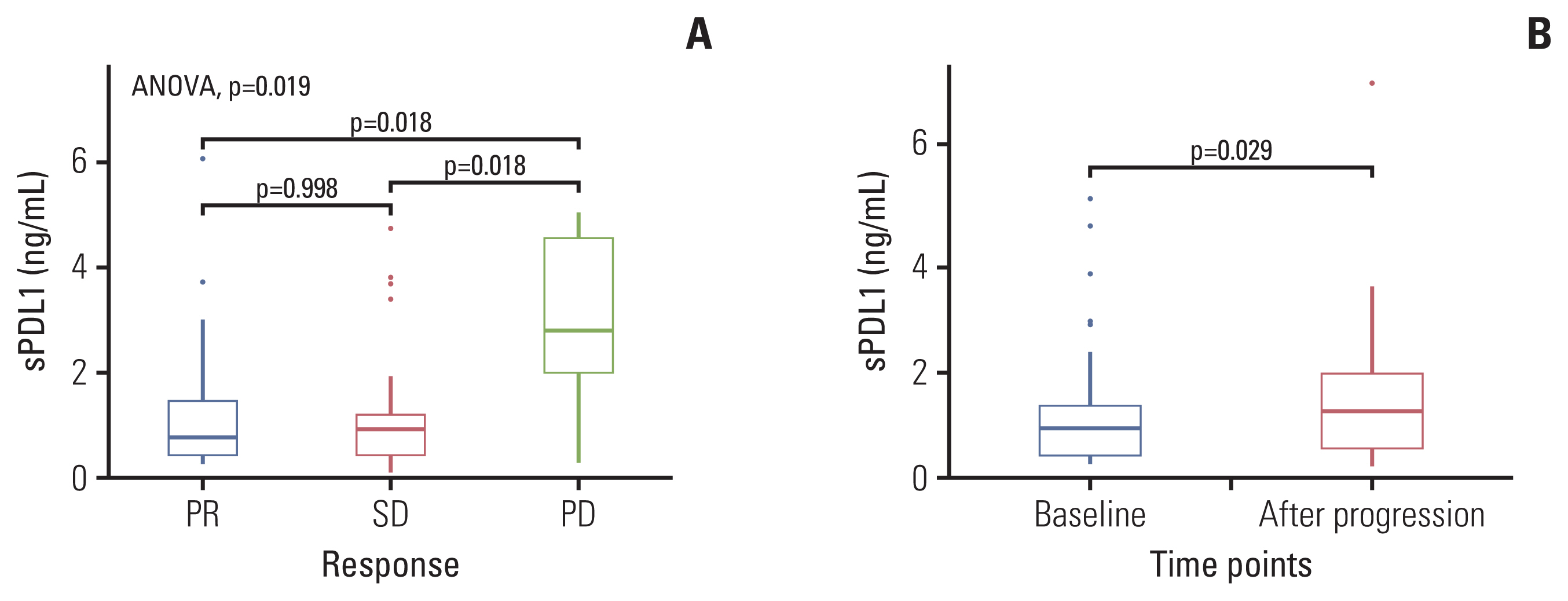
The median baseline sPDL1 was 0.8 ng/mL (range, 0.06 to 6.06 ng/mL). The median OS and PFS were 14.9 months and 8.0 months, respectively. sPDL1 and NLR showed a weak positive correlation (Spearman’s rho=0.301, p=0.013). Patients with low levels of sPDL1 at diagnosis (< 1.92 ng/mL) showed a better OS and PFS than patients with a high sPDL1. The baseline sPDL1 before treatment was higher in the progressive disease group than in the stable disease and partial response groups. Patients whose sPDL1 increased after the first cycle of chemotherapy showed worse PFS and OS. Following disease progression, sPDL1 increased compared with the baseline.
Conclusion
sPDL1 at prechemotherapy confers a prognostic value for PFS and OS in GC patients under palliative first-line chemotherapy. Dynamics of sPDL1 during chemotherapy correlates with disease progression.
Figure
Reference
-
References
1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015; 136:E359–86.
Article2. Corley DA, Buffler PA. Oesophageal and gastric cardia adenocarcinomas: analysis of regional variation using the Cancer Incidence in Five Continents database. Int J Epidemiol. 2001; 30:1415–25.
Article3. Kim YH, Shin SW, Kim BS, Kim JH, Kim JG, Mok YJ, et al. Paclitaxel, 5-fluorouracil, and cisplatin combination chemotherapy for the treatment of advanced gastric carcinoma. Cancer. 1999; 85:295–301.
Article4. Bang YJ, Kang WK, Kang YK, Kim HC, Jacques C, Zuber E, et al. Docetaxel 75 mg/m(2) is active and well tolerated in patients with metastatic or recurrent gastric cancer: a phase II trial. Jpn J Clin Oncol. 2002; 32:248–54.
Article5. Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010; 376:687–97.
Article6. Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014; 383:31–9.
Article7. U.S. Food and Drug Administration. FDA grants accelerated approval to pembrolizumab for advanced gastric cancer. Silver Spring MD: U.S. Food and Drug Administration;2018.8. Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017; 390:2461–71.
Article9. Doi T, Piha-Paul SA, Jalal SI, Saraf S, Lunceford J, Koshiji M, et al. Safety and antitumor activity of the anti-programmed death-1 antibody pembrolizumab in patients with advanced esophageal carcinoma. J Clin Oncol. 2018; 36:61–7.
Article10. Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, et al. Signatures of mutational processes in human cancer. Nature. 2013; 500:415–21.11. Zhang J, Gao J, Li Y, Nie J, Dai L, Hu W, et al. Circulating PD-L1 in NSCLC patients and the correlation between the level of PD-L1 expression and the clinical characteristics. Thorac Cancer. 2015; 6:534–8.12. Finkelmeier F, Canli O, Tal A, Pleli T, Trojan J, Schmidt M, et al. High levels of the soluble programmed death-ligand (sPD-L1) identify hepatocellular carcinoma patients with a poor prognosis. Eur J Cancer. 2016; 59:152–9.
Article13. Ha H, Nam AR, Bang JH, Park JE, Kim TY, Lee KH, et al. Soluble programmed death-ligand 1 (sPDL1) and neutrophil-to-lymphocyte ratio (NLR) predicts survival in advanced biliary tract cancer patients treated with palliative chemotherapy. Oncotarget. 2016; 7:76604–12.
Article14. Okuma Y, Hosomi Y, Nakahara Y, Watanabe K, Sagawa Y, Homma S. High plasma levels of soluble programmed cell death ligand 1 are prognostic for reduced survival in advan-ced lung cancer. Lung Cancer. 2017; 104:1–6.
Article15. Zheng Z, Bu Z, Liu X, Zhang L, Li Z, Wu A, et al. Level of circulating PD-L1 expression in patients with advanced gastric cancer and its clinical implications. Chin J Cancer Res. 2014; 26:104–11.16. Takahashi N, Iwasa S, Sasaki Y, Shoji H, Honma Y, Takashima A, et al. Serum levels of soluble programmed cell death ligand 1 as a prognostic factor on the first-line treatment of metastatic or recurrent gastric cancer. J Cancer Res Clin Oncol. 2016; 142:1727–38.
Article17. Pencina MJ, D’Agostino RB Sr. Evaluating discrimination of risk prediction models: the C statistic. JAMA. 2015; 314:1063–4.18. Ha H, Bang JH, Nam AR, Park JE, Jin MH, Bang YJ, et al. Dynamics of soluble programmed death-ligand 1 (sPDL1) during chemotherapy and its prognostic implications in cancer patients: biomarker development in immuno-oncology. Cancer Res Treat. 2019; 51:832–40.
Article19. Park H, Bang JH, Nam AR, Eun Park J, Hua Jin M, Bang YJ, et al. Prognostic implications of soluble programmed death-ligand 1 and its dynamics during chemotherapy in unresectable pancreatic cancer. Sci Rep. 2019; 9:11131.
Article20. Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002; 8:793–800.
Article21. Zhou J, Mahoney KM, Giobbie-Hurder A, Zhao F, Lee S, Liao X, et al. Soluble PD-L1 as a biomarker in malignant melanoma treated with checkpoint blockade. Cancer Immunol Res. 2017; 5:480–92.
Article22. Daassi D, Mahoney KM, Freeman GJ. The importance of exosomal PDL1 in tumour immune evasion. Nat Rev Immunol. 2020; 20:209–15.23. Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008; 26:677–704.
Article24. Frigola X, Inman BA, Krco CJ, Liu X, Harrington SM, Bulur PA, et al. Soluble B7-H1: differences in production between dendritic cells and T cells. Immunol Lett. 2012; 142:78–82.
Article25. Echizen K, Hirose O, Maeda Y, Oshima M. Inflammation in gastric cancer: interplay of the COX-2/prostaglandin E2 and Toll-like receptor/MyD88 pathways. Cancer Sci. 2016; 107:391–7.26. Oh BS, Jang JW, Kwon JH, You CR, Chung KW, Kay CS, et al. Prognostic value of C-reactive protein and neutrophil-to-lymphocyte ratio in patients with hepatocellular carcinoma. BMC Cancer. 2013; 13:78.
Article27. Yin Y, Wang J, Wang X, Gu L, Pei H, Kuai S, et al. Prognostic value of the neutrophil to lymphocyte ratio in lung cancer: a meta-analysis. Clinics (Sao Paulo). 2015; 70:524–30.
Article28. Haram A, Boland MR, Kelly ME, Bolger JC, Waldron RM, Kerin MJ. The prognostic value of neutrophil-to-lymphocyte ratio in colorectal cancer: a systematic review. J Surg Oncol. 2017; 115:470–9.
Article29. Ock CY, Nam AR, Lee J, Bang JH, Lee KH, Han SW, et al. Prognostic implication of antitumor immunity measured by the neutrophil-lymphocyte ratio and serum cytokines and angiogenic factors in gastric cancer. Gastric Cancer. 2017; 20:254–62.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recent Progress in Immunotherapy for Advanced Gastric Cancer
- Dynamics of Soluble Programmed Death-Ligand 1 (sPDL1) during Chemotherapy and Its Prognostic Implications in Cancer Patients: Biomarker Development in Immuno-oncology
- An update on immunotherapy with PD-1 and PD-L1 blockade
- Gastrointestinal cancer treatment with immune checkpoint inhibitors
- Cancer Immunotherapy Related Endocrine Adverse Effects