Cancer Res Treat.
2021 Jan;53(1):131-139. 10.4143/crt.2020.330.
Systemic Inflammatory Biomarkers, Especially Fibrinogen to Albumin Ratio, Predict Prognosis in Patients with Pancreatic Cancer
- Affiliations
-
- 1Department of Gastrointestinal Medical Oncology, Harbin Medical University Cancer Hospital, Harbin, China
- 2Translational Medicine Research and Cooperation Center of Northern China, Heilongjiang Academy of Medical Sciences, Harbin, China
- 3Department of Hematopathology, Anshan Hospital, The First Affiliated Hospital of China Medical University, Anshan, China
- KMID: 2510655
- DOI: http://doi.org/10.4143/crt.2020.330
Abstract
- Purpose
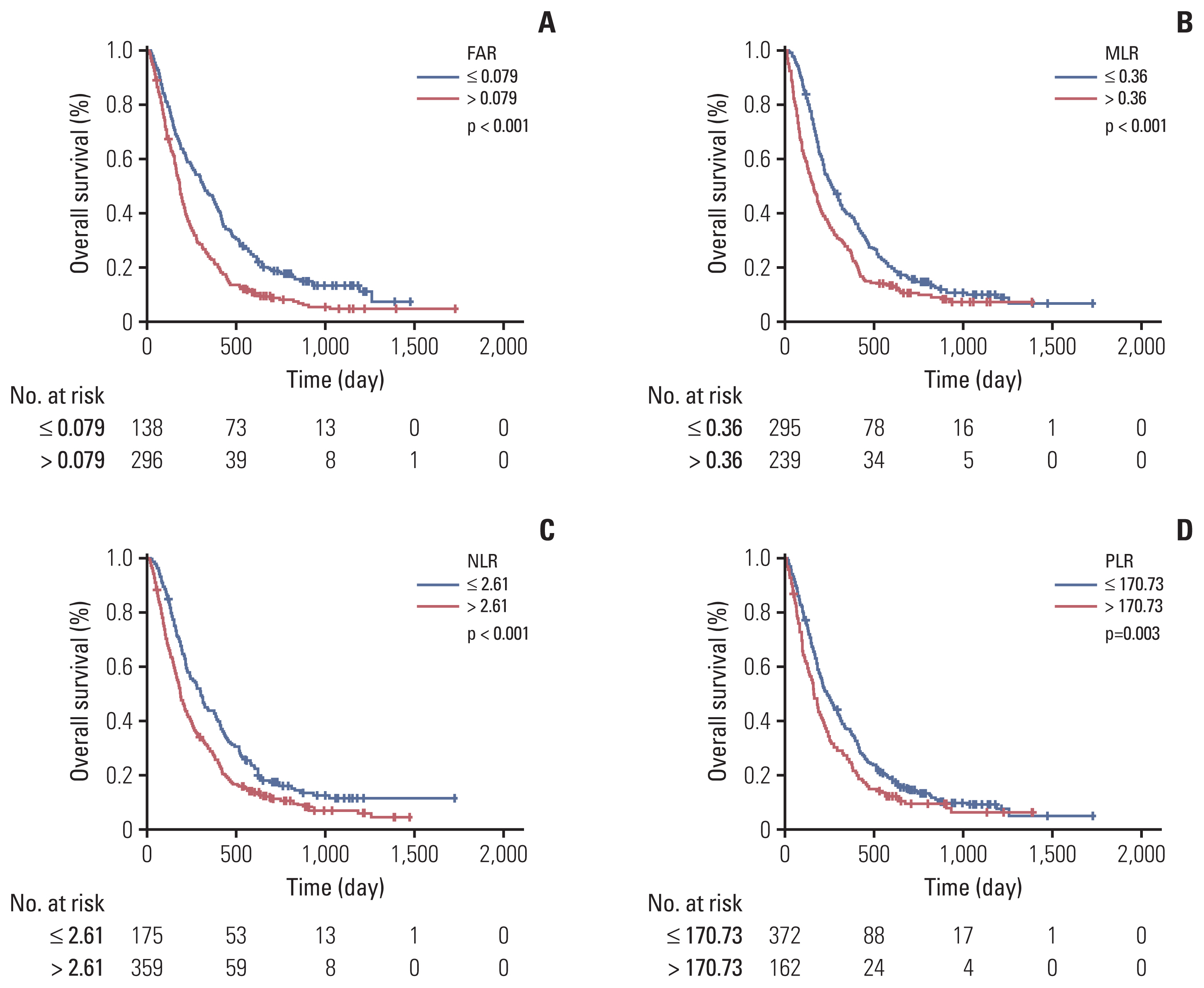
Systemic inflammatory response is a critical factor that promotes the initiation and metastasis of malignancies including pancreatic cancer (PC). This study was designed to determine and compare the prognostic value of neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), monocyte-to-lymphocyte ratio (MLR), and fibrinogen-to-albumin ratio (FAR) in resectable PC and locally advanced or metastatic PC.
Materials and Methods
Three hundred fifty-three patients with resectable PC and 807 patients with locally advan-ced or metastatic PC were recruited in this study. These patients were classified into a training set (n=758) and a validation set (n=402). Kaplan-Meier survival plots and Cox proportional hazards regression models were used to analyze prognosis.
Results
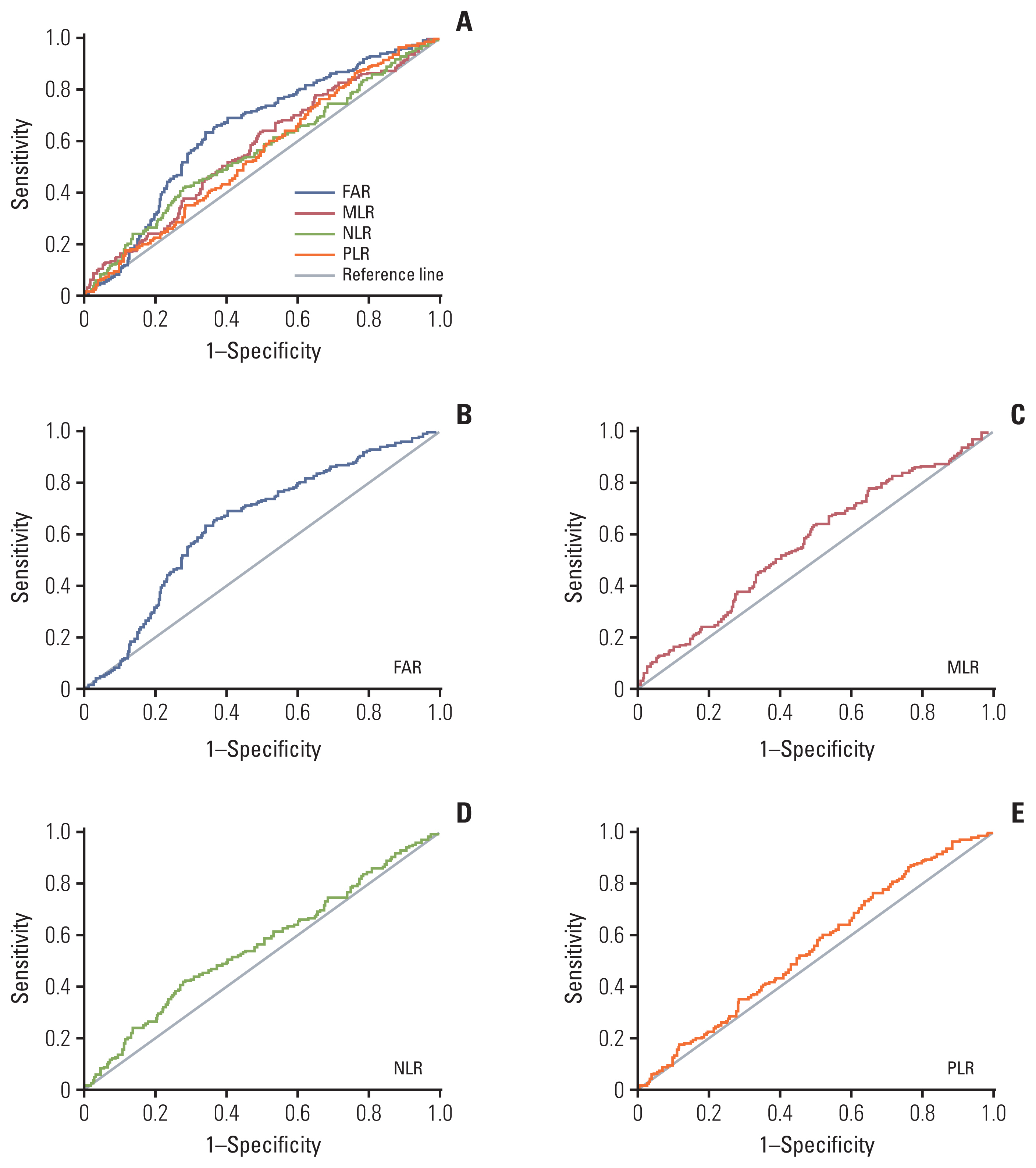
Overall survival (OS) was significantly better for patients with resectable PC with low preoperative PLR (p=0.048) and MLR (p=0.027). Low FAR, MLR, NLR (p < 0.001), and PLR (p=0.003) were significantly associated with decreased risk of death for locally advanced or metastatic PC patients. FAR (hazard ratio [HR], 1.522; 95% confidential interval [CI], 1.261 to 1.837; p < 0.001) and MLR (HR, 1.248; 95% CI, 1.017 to 1.532; p=0.034) were independent prognostic factors for locally advanced or metastatic PC.
Conclusion
The prognostic roles of FAR, MLR, NLR, and PLR in resectable PC and locally advanced or metastatic PC were different. FAR showed the most prognostic power in locally advanced or metastatic PC. Low FAR was positively correlated with OS in locally advanced or metastatic PC, which could be used to predict the prognosis.
Keyword
Figure
Reference
-
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019; 69:7–34.
Article2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424.
Article3. Witkowski ER, Smith JK, Tseng JF. Outcomes following resection of pancreatic cancer. J Surg Oncol. 2013; 107:97–103.
Article4. Cook MB, Barnett MJ, Bock CH, Cross AJ, Goodman PJ, Goodman GE, et al. Prediagnostic circulating markers of inflammation and risk of oesophageal adenocarcinoma: a study within the National Cancer Institute Cohort Consortium. Gut. 2019; 68:960–8.
Article5. Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer. 2016; 16:431–46.
Article6. Ku JH, Kang M, Kim HS, Jeong CW, Kwak C, Kim HH. The prognostic value of pretreatment of systemic inflammatory responses in patients with urothelial carcinoma undergoing radical cystectomy. Br J Cancer. 2015; 112:461–7.
Article7. Huang SH, Waldron JN, Milosevic M, Shen X, Ringash J, Su J, et al. Prognostic value of pretreatment circulating neutrophils, monocytes, and lymphocytes in oropharyngeal cancer stratified by human papillomavirus status. Cancer. 2015; 121:545–55.
Article8. Perisanidis C, Psyrri A, Cohen EE, Engelmann J, Heinze G, Perisanidis B, et al. Prognostic role of pretreatment plasma fibrinogen in patients with solid tumors: a systematic review and meta-analysis. Cancer Treat Rev. 2015; 41:960–70.
Article9. Mei Z, Shi L, Wang B, Yang J, Xiao Z, Du P, et al. Prognostic role of pretreatment blood neutrophil-to-lymphocyte ratio in advanced cancer survivors: a systematic review and meta-analysis of 66 cohort studies. Cancer Treat Rev. 2017; 58:1–13.
Article10. Sierzega M, Lenart M, Rutkowska M, Surman M, Mytar B, Matyja A, et al. Preoperative neutrophil-lymphocyte and lymphocyte-monocyte ratios reflect immune cell population rearrangement in resectable pancreatic cancer. Ann Surg Oncol. 2017; 24:808–15.
Article11. Tan Z, Zhang M, Han Q, Wen J, Luo K, Lin P, et al. A novel blood tool of cancer prognosis in esophageal squamous cell carcinoma: the Fibrinogen/Albumin Ratio. J Cancer. 2017; 8:1025–9.
Article12. Zou YX, Qiao J, Zhu HY, Lu RN, Xia Y, Cao L, et al. Albumin-to-fibrinogen ratio as an independent prognostic parameter in untreated chronic lymphocytic leukemia: a retrospective study of 191 cases. Cancer Res Treat. 2019; 51:664–71.
Article13. Liu JX, Li A, Zhou LY, Liu XF, Wei ZH, Wang XZ, et al. Significance of combined preoperative serum Alb and dNLR for diagnosis of pancreatic cancer. Future Oncol. 2018; 14:229–39.
Article14. Stotz M, Szkandera J, Stojakovic T, Seidel J, Samonigg H, Kornprat P, et al. The lymphocyte to monocyte ratio in peripheral blood represents a novel prognostic marker in patients with pancreatic cancer. Clin Chem Lab Med. 2015; 53:499–506.
Article15. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011; 144:646–74.16. Raucci MG, Fasolino I, Caporali M, Serrano-Ruiz M, Soriente A, Peruzzini M, et al. Exfoliated black phosphorus promotes in vitro bone regeneration and suppresses osteosarcoma progression through cancer-related inflammation inhibition. ACS Appl Mater Interfaces. 2019; 11:9333–42.
Article17. Stone ML, Beatty GL. Cellular determinants and therapeutic implications of inflammation in pancreatic cancer. Pharmacol Ther. 2019; 201:202–13.
Article18. Qi Q, Geng Y, Sun M, Wang P, Chen Z. Clinical implications of systemic inflammatory response markers as independent prognostic factors for advanced pancreatic cancer. Pancreatology. 2015; 15:145–50.
Article19. Trinchieri G. Cancer immunity: lessons from infectious diseases. J Infect Dis. 2015; 212(Suppl 1):S67–73.
Article20. Simpson-Haidaris PJ, Rybarczyk B. Tumors and fibrinogen: the role of fibrinogen as an extracellular matrix protein. Ann N Y Acad Sci. 2001; 936:406–25.21. Zhang F, Wang Y, Sun P, Wang ZQ, Wang DS, Zhang DS, et al. Fibrinogen promotes malignant biological tumor behavior involving epithelial-mesenchymal transition via the p-AKT/p-mTOR pathway in esophageal squamous cell carcinoma. J Cancer Res Clin Oncol. 2017; 143:2413–24.
Article22. Shu YJ, Weng H, Bao RF, Wu XS, Ding Q, Cao Y, et al. Clinical and prognostic significance of preoperative plasma hyperfibrinogenemia in gallbladder cancer patients following surgical resection: a retrospective and in vitro study. BMC Cancer. 2014; 14:566.
Article23. Ridker PM, Howard CP, Walter V, Everett B, Libby P, Hensen J, et al. Effects of interleukin-1beta inhibition with canakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen: a phase IIb randomized, placebo-controlled trial. Circulation. 2012; 126:2739–48.24. Ataseven B, du Bois A, Reinthaller A, Traut A, Heitz F, Aust S, et al. Pre-operative serum albumin is associated with post-operative complication rate and overall survival in patients with epithelial ovarian cancer undergoing cytoreductive surgery. Gynecol Oncol. 2015; 138:560–5.
Article25. Rothschild MA, Oratz M, Schreiber SS. Serum albumin. Hepatology. 1988; 8:385–401.
Article26. Bairey O, Shacham-Abulafia A, Shpilberg O, Gurion R. Serum albumin level at diagnosis of diffuse large B-cell lymphoma: an important simple prognostic factor. Hematol Oncol. 2016; 34:184–92.
Article27. Antkowiak M, Gabr A, Das A, Ali R, Kulik L, Ganger D, et al. Prognostic role of albumin, bilirubin, and ALBI scores: analysis of 1000 patients with hepatocellular carcinoma undergoing radioembolization. Cancers (Basel). 2019; 11:879.
Article28. Qi Q, Geng Y, Sun M, Chen H, Wang P, Chen Z. Hyperfibrinogen is associated with the systemic inflammatory response and predicts poor prognosis in advanced pancreatic cancer. Pancreas. 2015; 44:977–82.
Article29. Li SQ, Jiang YH, Lin J, Zhang J, Sun F, Gao QF, et al. Albumin-to-fibrinogen ratio as a promising biomarker to predict clinical outcome of non-small cell lung cancer individuals. Cancer Med. 2018; 7:1221–31.
Article30. Liang Y, Wang W, Que Y, Guan Y, Xiao W, Fang C, et al. Prognostic value of the fibrinogen/albumin ratio (FAR) in patients with operable soft tissue sarcoma. BMC Cancer. 2018; 18:942.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Fibrinogen to Mean Platelet Volume Ratio Can Predict Overall Survival of Patients with Non-Metastatic Gastric Cancer
- Prognostic Influence of Preoperative Fibrinogen to Albumin Ratio for Breast Cancer
- Inflammatory Response Markers as Predictors of Colorectal Cancer Prognosis
- Albumin-to-Fibrinogen Ratio as an Independent Prognostic Parameter in Untreated Chronic Lymphocytic Leukemia: A Retrospective Study of 191 Cases
- Assessment of Asymptomatically Increased CA 19-9