Acute Crit Care.
2020 Nov;35(4):286-295. 10.4266/acc.2020.00304.
Therapeutic hypothermia reduces inflammation and oxidative stress in the liver after asphyxial cardiac arrest in rats
- Affiliations
-
- 1Department of Emergency Medicine, Kangwon National University Hospital, School of Medicine, Kangwon National University, Chuncheon, Korea
- 2Department of Physical Therapy, College of Health Science, Youngsan University, Yangsan, Korea
- 3Department of Neurobiology, School of Medicine, Kangwon National University, Chuncheon, Korea
- 4Department of Biomedical Science and Research Institute for Bioscience and Biotechnology, Hallym University, Chuncheon, Korea
- 5Bio-Safety Research Institute, College of Veterinary Medicine, Chonbuk National University, Iksan, Korea
- 6Department of Anatomy, College of Korean Medicine, Dongguk University, Gyeongju, Korea
- KMID: 2510548
- DOI: http://doi.org/10.4266/acc.2020.00304
Abstract
- Background
Few studies have evaluated the effects of hypothermia on cardiac arrest (CA)-induced liver damage. This study aimed to investigate the effects of hypothermic therapy on the liver in a rat model of asphyxial cardiac arrest (ACA).
Methods
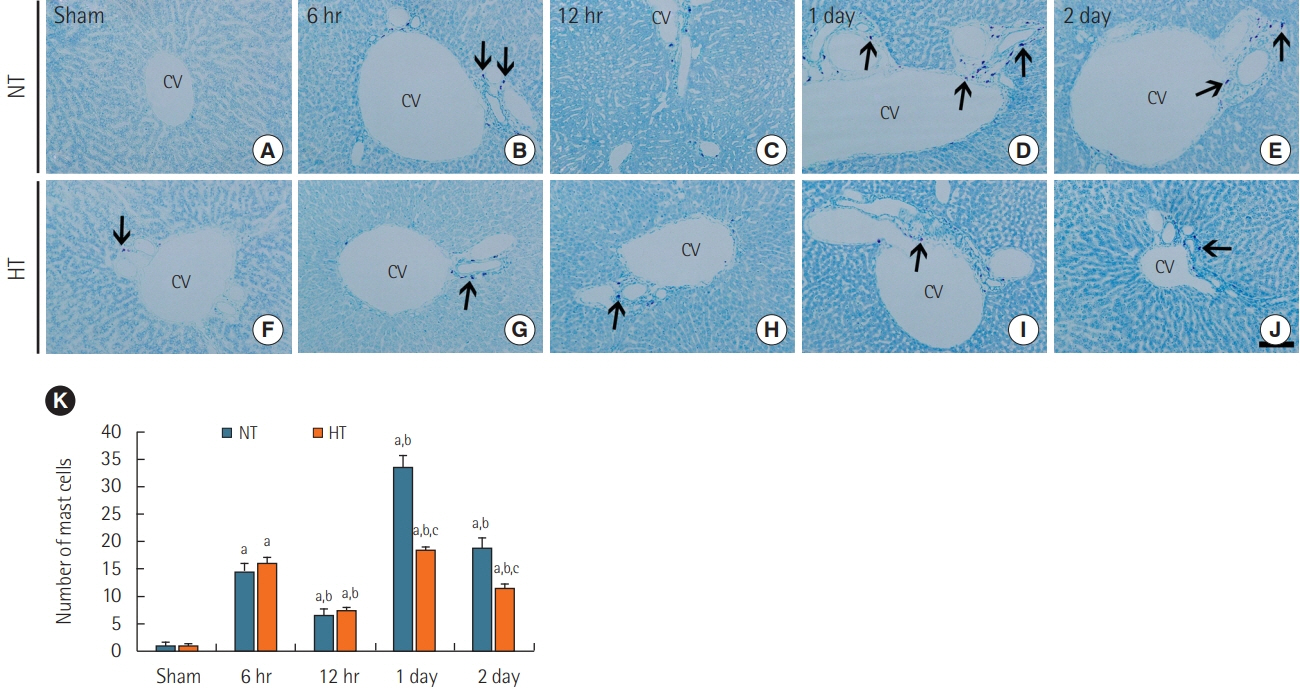
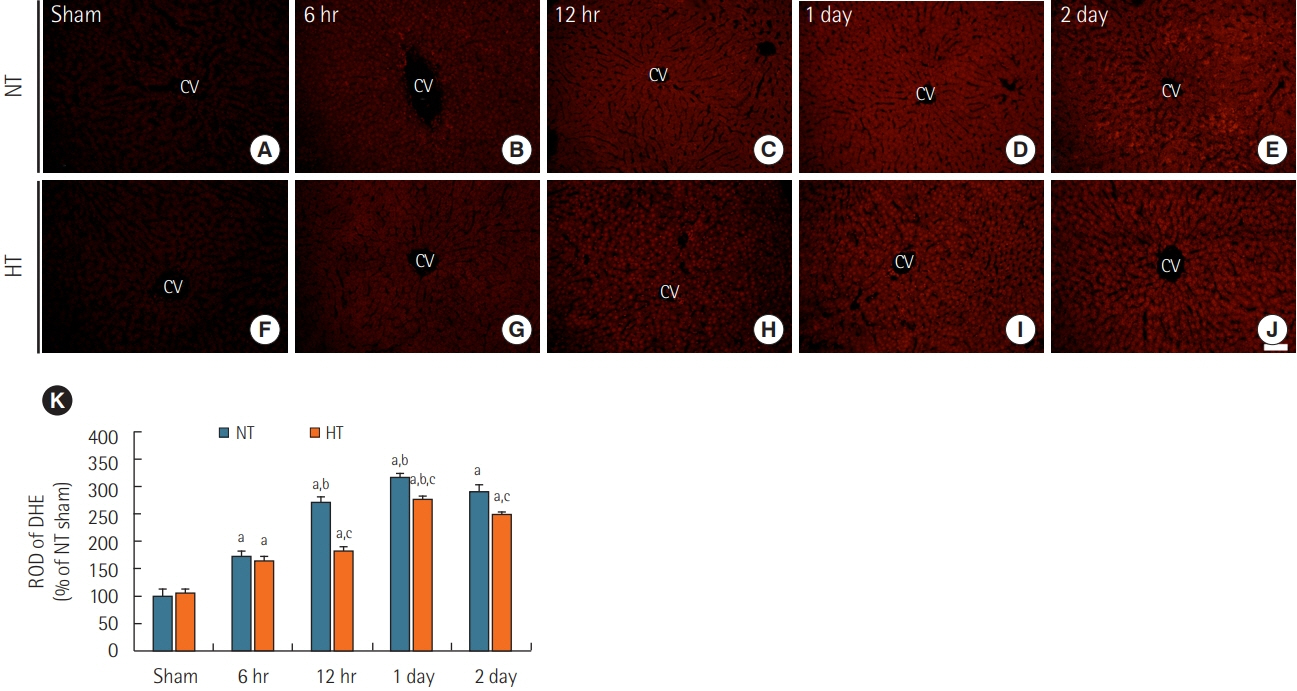
Rats were subjected to 5-minute ACA followed by return of spontaneous circulation (RoSC). Body temperature was controlled at 33°C±0.5°C or 37°C±0.5°C for 4 hours after RoSC in the hypothermia group and normothermia group, respectively. Liver tissues in each group were collected at 6 hours, 12 hours, 1 day, and 2 days after RoSC. To examine hepatic inflammation, mast cells were stained with toluidine blue. Superoxide anion radical production was evaluated using dihydroethidium fluorescence straining and expression of endogenous antioxidants (superoxide dismutase 1 [SOD1] and SOD2) was examined using immunohistochemistry.
Results
There were significantly more mast cells in the livers of the normothermia group with ACA than in the hypothermia group with ACA. Gradual increase in superoxide anion radical production was found with time in the normothermia group with ACA, but production was significantly suppressed in the hypothermia group with ACA relative to the normothermia group with ACA. SOD1 and SOD2 levels were higher in the hypothermia group with ACA than in the normothermia group with ACA.
Conclusions
Experimental hypothermic treatment after ACA significantly inhibited inflammation and superoxide anion radical production in the rat liver, indicating that this treatment enhanced or maintained expression of antioxidants. Our findings suggest that hypothermic therapy after CA can reduce mast cell-mediated inflammation through regulation of oxidative stress and the expression of antioxidants in the liver.
Keyword
Figure
Reference
-
1. Jaeschke H, Woolbright BL. Current strategies to minimize hepatic ischemia-reperfusion injury by targeting reactive oxygen species. Transplant Rev (Orlando). 2012; 26:103–14.
Article2. Pan S, Liu L, Pan H, Ma Y, Wang D, Kang K, et al. Protective effects of hydroxytyrosol on liver ischemia/reperfusion injury in mice. Mol Nutr Food Res. 2013; 57:1218–27.
Article3. Alía M, Horcajo C, Bravo L, Goya L. Effect of grape antioxidant dietary fiber on the total antioxidant capacity and the activity of liver antioxidant enzymes in rats. Nutr Res. 2003; 23:1251–67.
Article4. Garnacho-Castaño MV, Alva N, Sánchez-Nuño S, Bardallo RG, Palomeque J, Carbonell T. Hypothermia can reverse hepatic oxidative stress damage induced by hypoxia in rats. J Physiol Biochem. 2016; 72:615–23.
Article5. Hackenhaar FS, Medeiros TM, Heemann FM, Behling CS, Putti JS, Mahl CD, et al. Therapeutic hypothermia reduces oxidative damage and alters antioxidant defenses after cardiac arrest. Oxid Med Cell Longev. 2017; 2017:8704352.
Article6. Rao J, Zhang C, Wang P, Lu L, Zhang F. All-trans retinoic acid alleviates hepatic ischemia/reperfusion injury by enhancing manganese superoxide dismutase in rats. Biol Pharm Bull. 2010; 33:869–75.
Article7. Glantzounis GK, Salacinski HJ, Yang W, Davidson BR, Seifalian AM. The contemporary role of antioxidant therapy in attenuating liver ischemia-reperfusion injury: a review. Liver Transpl. 2005; 11:1031–47.
Article8. Zhang YS, Liu B, Luo XJ, Zhang JJ, Li NS, Ma QL, et al. A novel function of nuclear nonmuscle myosin regulatory light chain in promotion of xanthine oxidase transcription after myocardial ischemia/reperfusion. Free Radic Biol Med. 2015; 83:115–28.
Article9. Hackenhaar FS, Fumagalli F, Li Volti G, Sorrenti V, Russo I, Staszewsky L, et al. Relationship between post-cardiac arrest myocardial oxidative stress and myocardial dysfunction in the rat. J Biomed Sci. 2014; 21:70.
Article10. Forrester SJ, Kikuchi DS, Hernandes MS, Xu Q, Griendling KK. Reactive oxygen species in metabolic and inflammatory signaling. Circ Res. 2018; 122:877–902.
Article11. Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012; 24:981–90.
Article12. Sehitoglu MH, Karaboga I, Kiraz A, Kiraz HA. The hepatoprotective effect of Aloe vera on ischemia-reperfusion injury in rats. North Clin Istanb. 2019; 6:203–9.13. Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002; 82:47–95.
Article14. Ighodaro OM, Akinloye OA. First line defence antioxidantssuperoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria J Med. 2018; 54:287–93.
Article15. Li Y, Ristagno G, Guan J, Barbut D, Bisera J, Weil MH, et al. Preserved heart rate variability during therapeutic hypothermia correlated to 96 hrs neurological outcomes and survival in a pig model of cardiac arrest. Crit Care Med. 2012; 40:580–6.
Article16. Testori C, Sterz F, Behringer W, Haugk M, Uray T, Zeiner A, et al. Mild therapeutic hypothermia is associated with favourable outcome in patients after cardiac arrest with non-shockable rhythms. Resuscitation. 2011; 82:1162–7.
Article17. Gong P, Li CS, Hua R, Zhao H, Tang ZR, Mei X, et al. Mild hypothermia attenuates mitochondrial oxidative stress by protecting respiratory enzymes and upregulating MnSOD in a pig model of cardiac arrest. PLoS One. 2012; 7:e35313.
Article18. Ostadal P, Mlcek M, Kruger A, Horakova S, Skabradova M, Holy F, et al. Mild therapeutic hypothermia is superior to controlled normothermia for the maintenance of blood pressure and cerebral oxygenation, prevention of organ damage and suppression of oxidative stress after cardiac arrest in a porcine model. J Transl Med. 2013; 11:124.
Article19. Dohi K, Miyamoto K, Fukuda K, Nakamura S, Hayashi M, Ohtaki H, et al. Status of systemic oxidative stress during therapeutic hypothermia in patients with post-cardiac arrest syndrome. Oxid Med Cell Longev. 2013; 2013:562429.
Article20. Han F, Boller M, Guo W, Merchant RM, Lampe JW, Smith TM, et al. A rodent model of emergency cardiopulmonary bypass resuscitation with different temperatures after asphyxial cardiac arrest. Resuscitation. 2010; 81:93–9.
Article21. Drabek T, Foley LM, Janata A, Stezoski J, Hitchens TK, Manole MD, et al. Global and regional differences in cerebral blood flow after asphyxia versus ventricular fibrillation cardiac arrest in rats using ASL-MRI. Resuscitation. 2014; 85:964–71.22. Laubach VE, Sharma AK. Mechanisms of lung ischemia-reperfusion injury. Curr Opin Organ Transplant. 2016; 21:246–52.
Article23. Girotra S, Chan PS, Bradley SM. Post-resuscitation care following out-of-hospital and in-hospital cardiac arrest. Heart. 2015; 101:1943–9.
Article24. Lee JC, Tae HJ, Cho JH, Kim IS, Lee TK, Park CW, et al. Therapeutic hypothermia attenuates paraplegia and neuronal damage in the lumbar spinal cord in a rat model of asphyxia cardiac arrest. J Therm Biol. 2019; 83:1–7.25. Jia X, Koenig MA, Shin HC, Zhen G, Pardo CA, Hanley DF, et al. Improving neurological outcomes post-cardiac arrest in a rat model: immediate hypothermia and quantitative EEG monitoring. Resuscitation. 2008; 76:431–42.
Article26. Lee CH, Park JH, Cho JH, Kim IH, Ahn JH, Lee JC, et al. Effect of Oenanthe javanica extract on antioxidant enzyme in the rat liver. Chin Med J (Engl). 2015; 128:1649–54.
Article27. Hargrove L, Graf-Eaton A, Kennedy L, Demieville J, Owens J, Hodges K, et al. Isolation and characterization of hepatic mast cells from cholestatic rats. Lab Invest. 2016; 96:1198–210.
Article28. Yang F, Shang L, Wang S, Liu Y, Ren H, Zhu W, et al. TNF α-mediated necroptosis aggravates ischemia-reperfusion injury in the fatty liver by regulating the inflammatory response. Oxid Med Cell Longev. 2019; 2019:2301903.29. Park JH, Park Ok, Cho JH, Chen BH, Kim IH, Ahn JH, et al. Anti-inflammatory effect of tanshinone I in neuroprotection against cerebral ischemia-reperfusion injury in the gerbil hippocampus. Neurochem Res. 2014; 39:1300–12.
Article30. da Silva EZ, Jamur MC, Oliver C. Mast cell function: a new vision of an old cell. J Histochem Cytochem. 2014; 62:698–738.31. Moon TC, Befus AD, Kulka M. Mast cell mediators: their differential release and the secretory pathways involved. Front Immunol. 2014; 5:569.
Article32. Yang MQ, Ma YY, Tao SF, Ding J, Rao LH, Jiang H, et al. Mast cell degranulation promotes ischemia-reperfusion injury in rat liver. J Surg Res. 2014; 186:170–8.
Article33. Yang MQ, Ma YY, Ding J, Li JY. The role of mast cells in ischemia and reperfusion injury. Inflamm Res. 2014; 63:899–905.
Article34. Biran V, Cochois V, Karroubi A, Arrang JM, Charriaut-Marlangue C, Héron A. Stroke induces histamine accumulation and mast cell degranulation in the neonatal rat brain. Brain Pathol. 2008; 18:1–9.
Article35. Hei ZQ, Gan XL, Luo GJ, Li SR, Cai J. Pretreatment of cromolyn sodium prior to reperfusion attenuates early reperfusion injury after the small intestine ischemia in rats. World J Gastroenterol. 2007; 13:5139–46.
Article36. Abonia JP, Friend DS, Austen WG Jr, Moore FD Jr, Carroll MC, Chan R, et al. Mast cell protease 5 mediates ischemia-reperfusion injury of mouse skeletal muscle. J Immunol. 2005; 174:7285–91.
Article37. Boros M, Kaszaki J, Nagy S. Histamine release during intestinal ischemia-reperfusion: role of iron ions and hydrogen peroxide. Circ Shock. 1991; 35:174–80.38. Kurose I, Argenbright LW, Wolf R, Lianxi L, Granger DN. Ischemia/reperfusion-induced microvascular dysfunction: role of oxidants and lipid mediators. Am J Physiol. 1997; 272(6 Pt 2):H2976–82.
Article39. He Z, Ma C, Yu T, Song J, Leng J, Gu X, et al. Activation mechanisms and multifaceted effects of mast cells in ischemia reperfusion injury. Exp Cell Res. 2019; 376:227–35.
Article40. Zhao W, Gan X, Su G, Wanling G, Li S, Hei Z, et al. The interaction between oxidative stress and mast cell activation plays a role in acute lung injuries induced by intestinal ischemiareperfusion. J Surg Res. 2014; 187:542–52.
Article41. Selemidis S, Dusting GJ, Peshavariya H, Kemp-Harper BK, Drummond GR. Nitric oxide suppresses NADPH oxidase-dependent superoxide production by S-nitrosylation in human endothelial cells. Cardiovasc Res. 2007; 75:349–58.
Article42. Chen J, Rogers SC, Kavdia M. Analysis of kinetics of dihydroethidium fluorescence with superoxide using xanthine oxidase and hypoxanthine assay. Ann Biomed Eng. 2013; 41:327–37.
Article43. Kehrer JP, Lund LG. Cellular reducing equivalents and oxidative stress. Free Radic Biol Med. 1994; 17:65–75.
Article44. Abdel-Gaber SA, Ibrahim MA, Amin EF, Ibrahim SA, Mohammed RK, Abdelrahman AM. Effect of selective versus non-selective cyclooxygenase inhibitors on ischemia-reperfusion-induced hepatic injury in rats. Life Sci. 2015; 134:42–8.
Article45. Tekin IO, Sipahi EY, Comert M, Acikgoz S, Yurdakan G. Low-density lipoproteins oxidized after intestinal ischemia/reperfusion in rats. J Surg Res. 2009; 157:e47–54.
Article46. Ozkan H, Ekinci S, Uysal B, Akyildiz F, Turkkan S, Ersen O, et al. Evaluation and comparison of the effect of hypothermia and ozone on ischemia-reperfusion injury of skeletal muscle in rats. J Surg Res. 2015; 196:313–9.
Article47. Che D, Li L, Kopil CM, Liu Z, Guo W, Neumar RW. Impact of therapeutic hypothermia onset and duration on survival, neurologic function, and neurodegeneration after cardiac arrest. Crit Care Med. 2011; 39:1423–30.
Article48. Slikker W 3rd, Desai VG, Duhart H, Feuers R, Imam SZ. Hypothermia enhances bcl-2 expression and protects against oxidative stress-induced cell death in Chinese hamster ovary cells. Free Radic Biol Med. 2001; 31:405–11.
Article49. Khaliulin I, Clarke SJ, Lin H, Parker J, Suleiman MS, Halestrap AP. Temperature preconditioning of isolated rat hearts: a potent cardioprotective mechanism involving a reduction in oxidative stress and inhibition of the mitochondrial permeability transition pore. J Physiol. 2007; 581(Pt 3):1147–61.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Implementation of Therapeutic Hypothermia after Cardiac Arrest
- Effect of Mild Therapeutic Hypothermia in Comatose Survivors after Asphyxial Cardiac Arrest
- Hypothermic treatment reduces matrix metalloproteinase-9 expression and damage in the liver following asphyxial cardiac arrest in rats
- Implementation of Therapeutic Hypothermia after Pediatric Out-of Hospital Cardiac Arrest in One Tertiary Emergency Center
- The Effect of Mild Therapeutic Hypothermia on the non-Vf Cardiac arrest