Korean J Transplant.
2020 Dec;34(4):286-292. 10.4285/kjt.20.0023.
Pediatric split liver transplantation in a patient with biliary atresia polysplenia syndrome and agenesis of inferior vena cava
- Affiliations
-
- 1Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 2Department of Pediatrics, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2510268
- DOI: http://doi.org/10.4285/kjt.20.0023
Abstract
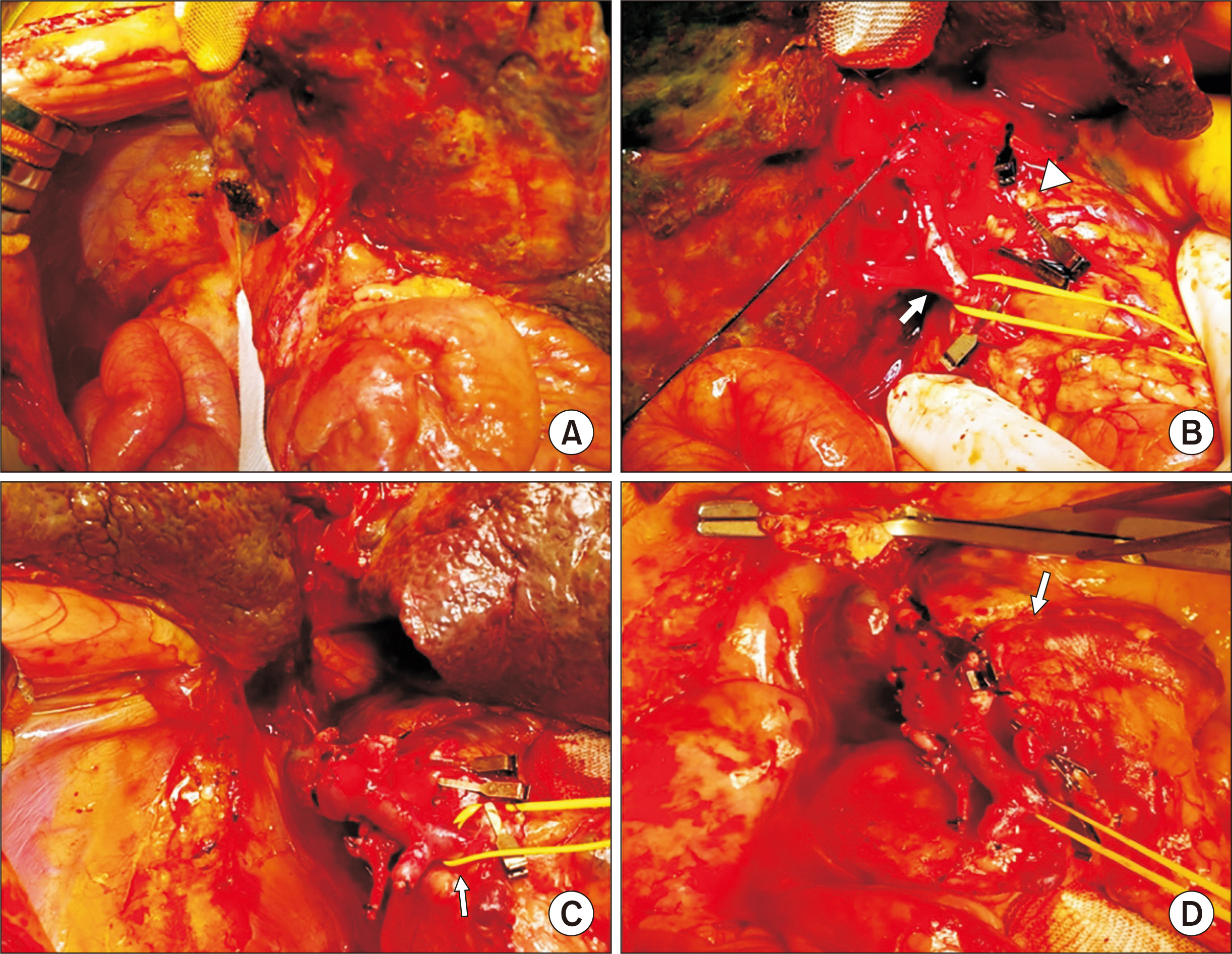
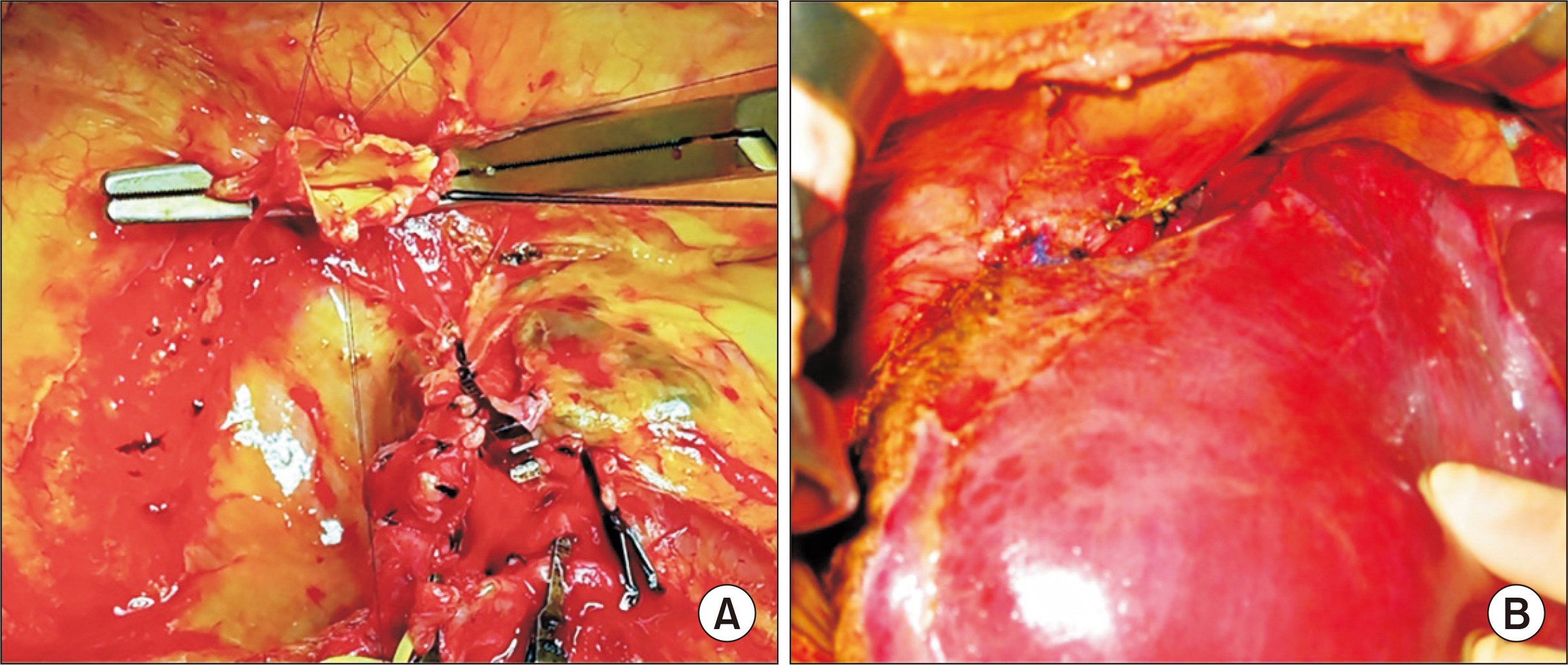
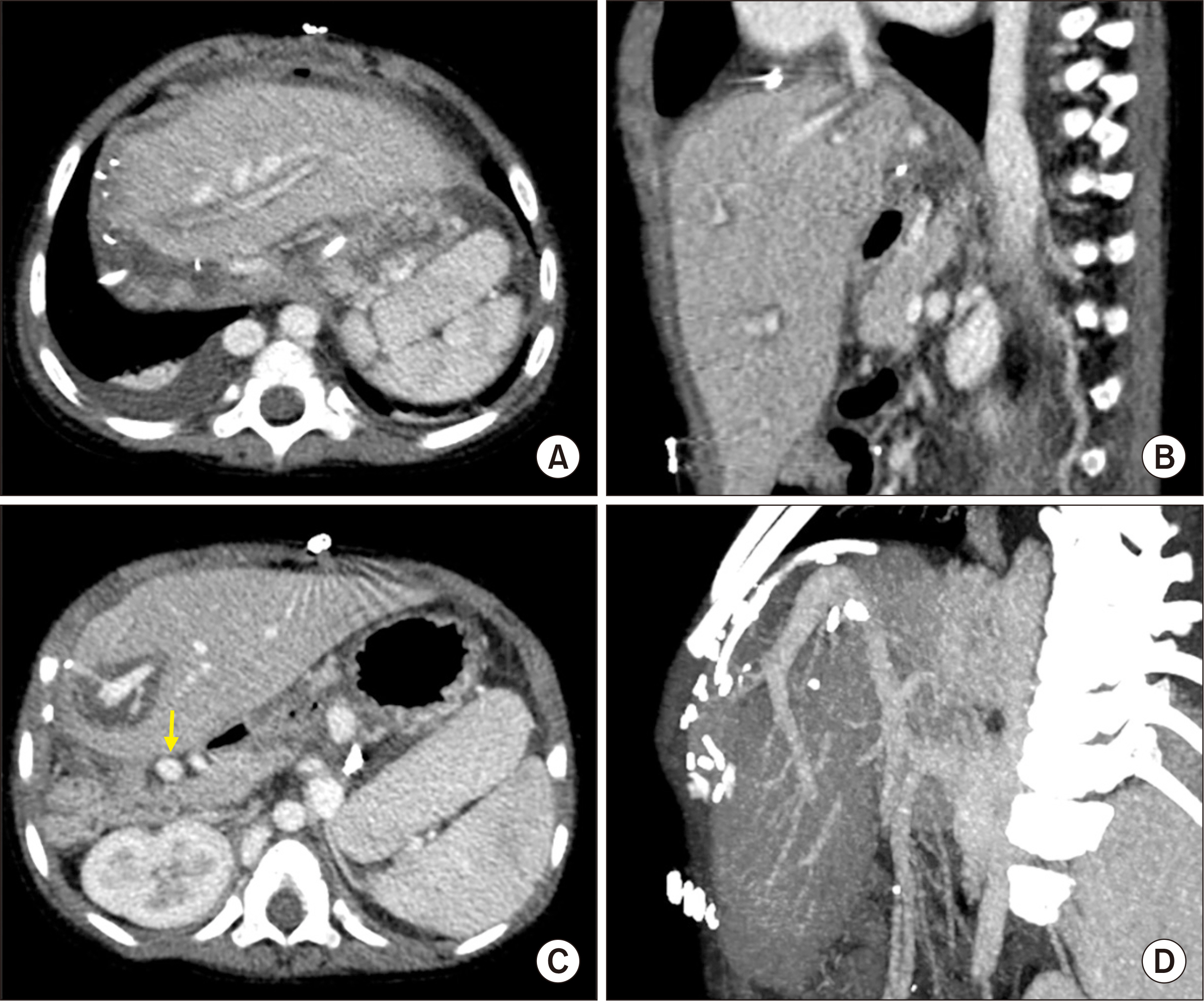
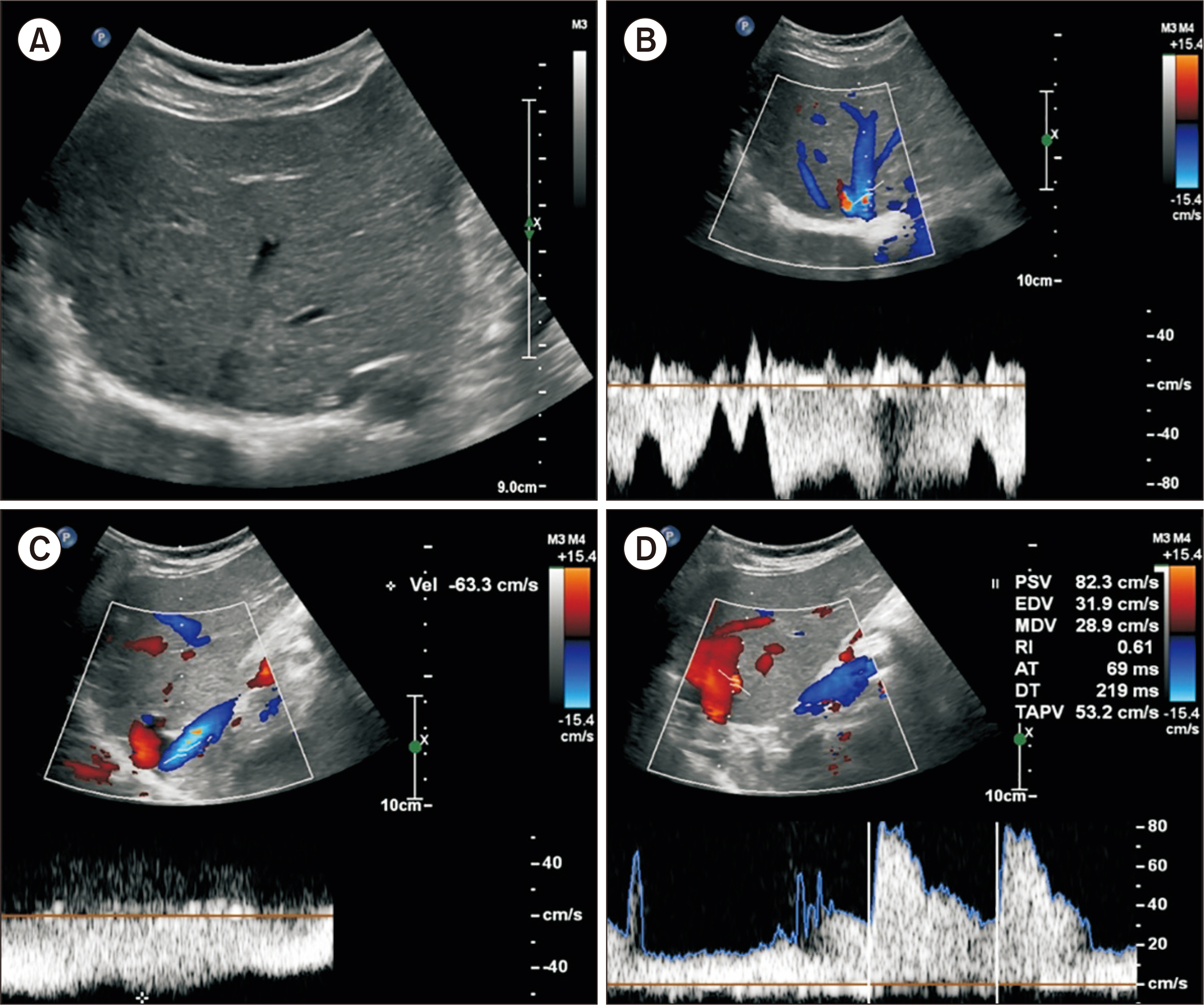
- Biliary atresia (BA)-polysplenia syndrome (PS) is diagnosed in a small proportion of BA patients. We present a case of split liver transplantation (LT) successfully performed in a pediatric recipient with BA-PS. The recipient was 29-month-old boy who underwent Kasai procedure. The coexisting malformations included agenesis of the inferior vena cava with azygos vein continuation, polysplenia, intestinal malrotation, truncated pan-creas, and preduodenal portal vein and annular pancreas. With pediatric end-stage liver disease score of 33, the patient was allocated for split LT. Under this condition, the left lateral section graft was equivalent to a graft-recipient weight ratio of 2.6%. The recipient surgery was performed according to the standard procedures of pediatric LT. The graft hepatic vein was directly anastomosed with the suprahepatic confluence of the recipient hepatic veins. An external iliac vein homograft was interposed for portal vein reconstruc- tion. Portal collateral veins were embolized intraoperatively to secure portal vein inflow. No surgical complications were developed. Currently, the patient has been doing well for 4 years after transplantation. Our pediatric patient with BA-PS had various anatomical malformations. Thorough preoperative and intraoperative assessment of these anoma- lies, adoption of customized reconstruction techniques of LT, and careful posttransplant monitoring are necessary for successful LT.
Keyword
Figure
Cited by 1 articles
-
Third retransplantation using a whole liver graft for late graft failure from hepatic vein stent stenosis in a pediatric patient who underwent split liver retransplantation
Jung-Man Namgoong, Shin Hwang, Young-In Yoon, Yong-Pil Cho, Woo-Hyoung Kang, Yong Jae Kwon, Hyunhee Kwon, Sang Hoon Kim, Kyung Mo Kim, Seak Hee Oh
Ann Hepatobiliary Pancreat Surg. 2021;25(2):299-306. doi: 10.14701/ahbps.2021.25.2.299.
Reference
-
1. Davenport M, Savage M, Mowat AP, Howard ER. 1993; Biliary atresia splenic malformation syndrome: an etiologic and prognostic subgroup. Surgery. 113:662–8. PMID: 8506525.2. Davenport M, Tizzard SA, Underhill J, Mieli-Vergani G, Portmann B, Hadzić N. 2006; The biliary atresia splenic malformation syndrome: a 28-year single-center retrospective study. J Pediatr. 149:393–400. DOI: 10.1016/j.jpeds.2006.05.030. PMID: 16939755.
Article3. Lilly JR, Starzl TE. 1974; Liver transplantation in children with biliary atresia and vascular anomalies. J Pediatr Surg. 9:707–14. DOI: 10.1016/0022-3468(74)90109-2. PMID: 4608947. PMCID: PMC2976667.4. Hoffman MA, Celli S, Ninkov P, Rolles K, Calne RY. 1989; Orthotopic transplantation of the liver in children with biliary atresia and polysplenia syndrome: report of two cases. J Pediatr Surg. 24:1020–2. DOI: 10.1016/S0022-3468(89)80206-4. PMID: 2809946.
Article5. Varela-Fascinetto G, Castaldo P, Fox IJ, Sudan D, Heffron TG, Shaw BW, et al. 1998; Biliary atresia-polysplenia syndrome: surgical and clinical relevance in liver transplantation. Ann Surg. 227:583–9. DOI: 10.1097/00000658-199804000-00022. PMID: 9563550. PMCID: PMC1191317.6. Broniszczak D, Apanasiewicz A, Czubkowski P, Kaliciński P, Ismail H, Ostoja-Chyzynska A, et al. 2011; Liver transplantation in children with biliary atresia and polysplenia syndrome. Ann Transplant. 16:14–7. DOI: 10.1016/0022-3468(91)90698-s. PMID: 2061802.7. Mattei P, Wise B, Schwarz K, Klein A, Colombani PM. 1998; Orthotopic liver transplantation in patients with biliary atresia and situs inversus. Pediatr Surg Int. 14:104–10. DOI: 10.1007/s003830050452. PMID: 9880714.
Article8. Ruíz P, Aguirre KR, Mesa CM, Lara LL. 2015; Case report of syndromic biliary atresia in a pediatric patient. Rev Col Gastroenterol. 30:452–6. DOI: 10.22516/25007440.9.9. Hartley JL, Davenport M, Kelly DA. 2009; Biliary atresia. Lancet. 374:1704–13. DOI: 10.1016/S0140-6736(09)60946-6. PMID: 19914515.
Article10. Feldman AG, Sokol RJ. 2013; Neonatal cholestasis. Neoreviews. 14:e63–73. DOI: 10.1542/neo.14-2-e63. PMID: 24244109. PMCID: PMC3827866.
Article11. Broniszczak D, Apanasiewicz A, Czubkowski P, Kaliciński P, Ismail H, Ostoja-Chyzynska A, et al. 2011; Liver transplantation in children with biliary atresia and polysplenia syndrome. Ann Transplant. 16:14–7. DOI: 10.1016/0022-3468(91)90698-s. PMID: 2061802.12. Hwang S, Kim DY, Ahn CS, Moon DB, Kim KM, Park GC, et al. 2013; Computational simulation-based vessel interposition reconstruction technique for portal vein hypoplasia in pediatric liver transplantation. Transplant Proc. 45:255–8. DOI: 10.1016/j.transproceed.2012.05.090. PMID: 23375311.
Article13. Kim JH, Ko GY, Sung KB, Yoon HK, Kim KR, Moon DB, et al. 2009; Transvenous variceal embolization during or after living-donor liver transplantation to improve portal venous flow. J Vasc Interv Radiol. 20:1454–9. DOI: 10.1016/j.jvir.2009.07.036. PMID: 19875063.
Article14. Nio M, Wada M, Sasaki H, Tanaka H, Watanabe T. 2015; Long-term outcomes of biliary atresia with splenic malformation. J Pediatr Surg. 50:2124–7. DOI: 10.1016/j.jpedsurg.2015.08.040. PMID: 26613836.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Absence of the Intrahepatic Inferior Vena Cava with Polysplenia Syndrome on Multidetector Computed Tomography: A Case Report
- Anomalies of Abdominal Organs in Polysplenia Syndrome: Multidetector Computed Tomography Findings
- Hemiazygos Continuation of Left Inferior Vena Cava Draining into the Right Atrium via Persistent Left Superior Vena Cava: A Variant of Polysplenia Syndrome Mimicking Aortic Dissection
- Combined Anomaly of the Right Hepatic Lobe Agenesis and Absence of the Inferior Vena Cava: a Case Report
- Inferior vena cava stenosis-induced sinusoidal obstructive syndrome after living donor liver transplantation