J Korean Med Sci.
2020 Dec;35(50):e420. 10.3346/jkms.2020.35.e420.
Expression of Estrogen Receptor-alpha in Nasal Polyps and the Effects of Dexamethasone on Estrogen Receptoralpha Expression in RPMI 2650 Cells
- Affiliations
-
- 1Department of Otorhinolaryngology-Head and Neck Surgery, Soonchunhyang University Cheonan Hospital, Soonchunhyang University College of Medicine, Cheonan, Korea
- 2Department of Biochemistry, Soonchunhyang University College of Medicine, Cheonan, Korea
- KMID: 2509754
- DOI: http://doi.org/10.3346/jkms.2020.35.e420
Abstract
- Background
Studies have reported that epithelial cell proliferation may be involved in the pathogenesis of nasal polyps (NPs). Estrogen receptor (ER)-α, one type of ER, is related to antiinflammatory action and cell survival in certain tissues. In this study, we examined the presence or absence of ER-α in NPs and healthy inferior turbinate mucosae. We also investigated the effect of dexamethasone on ER-α expression, cell viability, and apoptosis in RPMI 2650 cells.
Methods
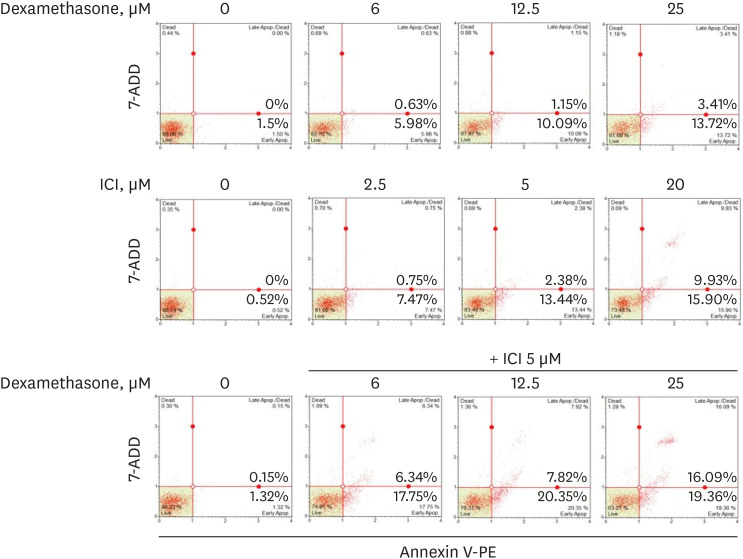
Immunohistochemical staining and Western blot analysis were conducted to determine the expression of ER-α in 15 NPs and 15 healthy inferior turbinate mucosae. After treating RPMI 2650 cells with dexamethasone, ER-α expression was analyzed using Western blot analysis and cell viability was determined using the MTT assay. Western blot analysis and annexin V-phycoerythrin (PE) staining were used to examine apoptotic cell death.
Results
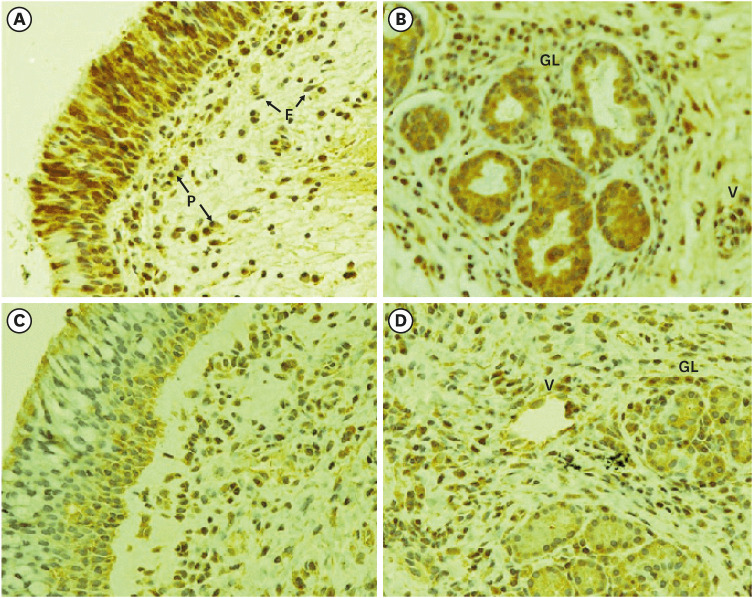
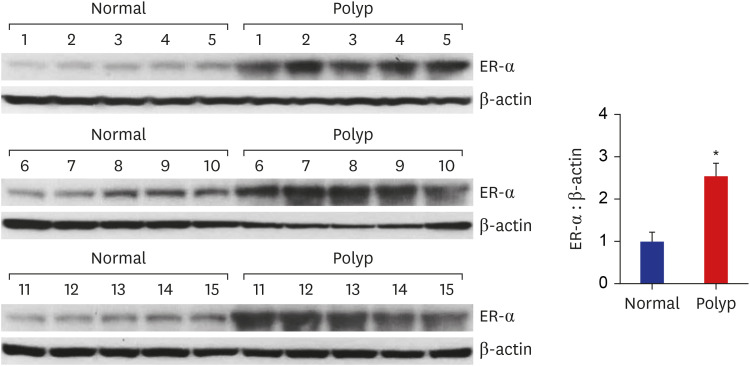
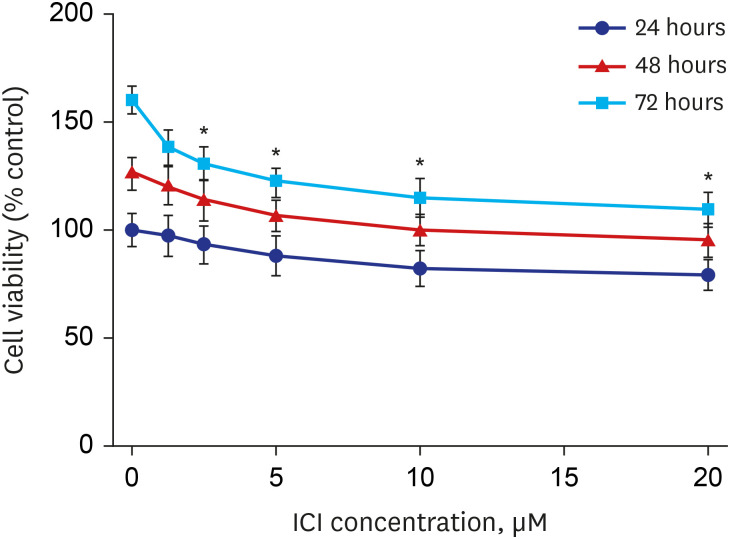
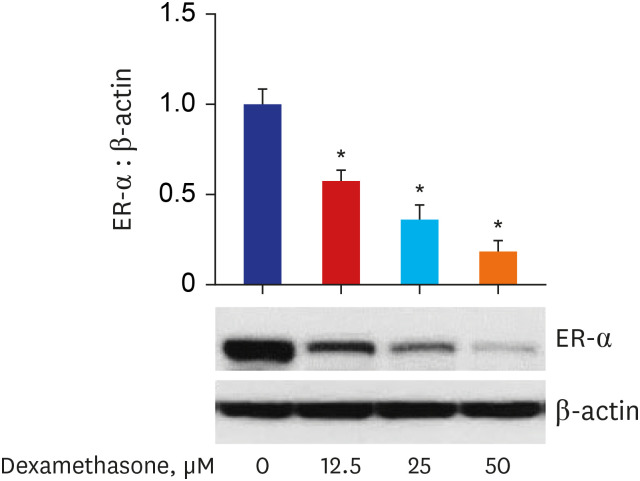
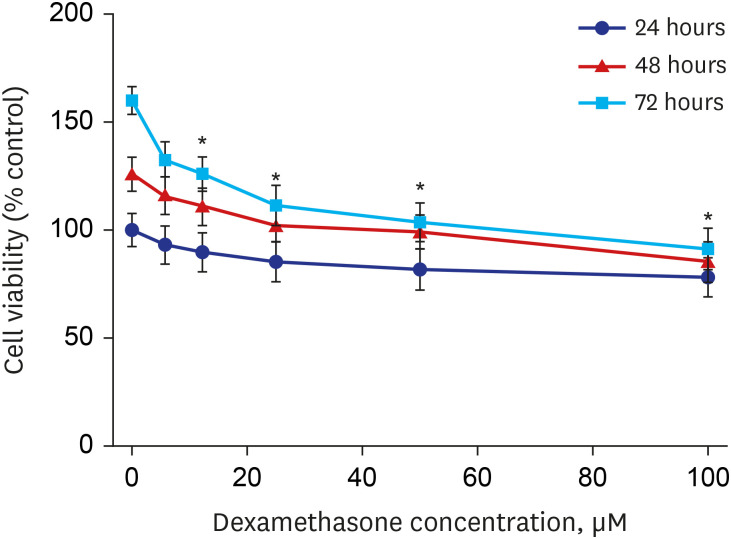
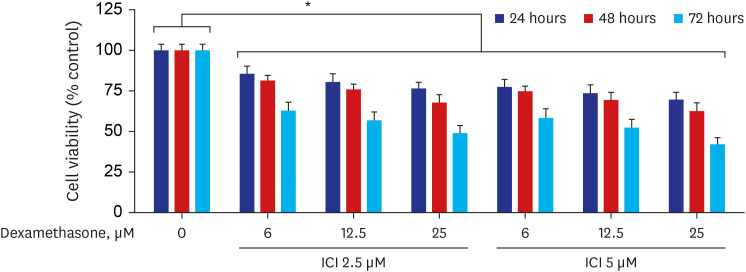
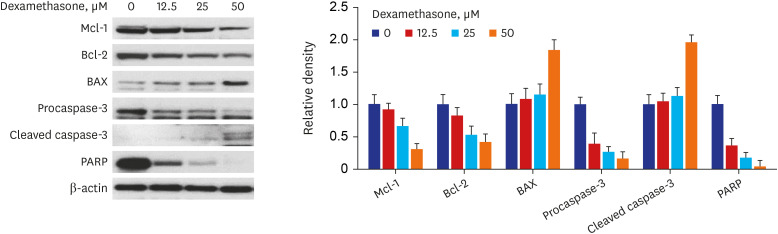
Western blot analysis showed that ER-α expression was upregulated in 13 of the 15 NP tissues. Immunohistochemical staining for ER-α confirmed the results of the Western blot analysis. When RPMI 2650 cells were treated with dexamethasone, both ER-α expression and cell viability were decreased. Furthermore, the treatment of RPMI 2650 cells with dexamethasone increased apoptotic cell death, as shown by increased levels of BAX and cleaved caspase-3, decreased levels of Bcl-2, and an increased percentage of positive annexin V-PE stained cells.
Conclusion
ER-α expression was higher in NPs than in healthy inferior turbinate mucosae. When RPMI 2650 cells were treated with dexamethasone, ER-α expression was downregulated, cell viability decreased, and apoptosis increased. The decreased cell viability may be related, at least in part, to the decreased ER-α protein levels, which likely contributed to the induction of apoptotic cell death in RPMI 2650 cells.
Figure
Reference
-
1. Settipane RA, Peters AT, Chandra R. Chapter 4: chronic rhinosinusitis. Am J Rhinol Allergy. 2013; 27(Suppl 1):S11–S15. PMID: 23711032.2. Coste A, Rateau JG, Bernaudin JF, Peynègre R, Escudier E. Nasal polyposis pathogenesis: a flow cytometric and immunohistochemical study of epithelial cell proliferation. Acta Otolaryngol. 1996; 116(5):755–761. PMID: 8908256.3. Fokkens W, Lund V, Mullol J. European Position Paper on Rhinosinusitis and Nasal Polyps Group. EP3OS 2007: European position paper on rhinosinusitis and nasal polyps 2007. A summary for otorhinolaryngologists. Rhinology. 2007; 45(2):97–101. PMID: 17708455.4. Watanabe K, Shirasaki H, Kanaizumi E, Himi T. Effects of glucocorticoids on infiltrating cells and epithelial cells of nasal polyps. Ann Otol Rhinol Laryngol. 2004; 113(6):465–473. PMID: 15224831.
Article5. Hirano S, Asano K, Namba M, Kanai K, Hisamitsu T, Suzaki H. Induction of apoptosis in nasal polyp fibroblasts by glucocorticoids in vitro. Acta Otolaryngol. 2003; 123(9):1075–1079. PMID: 14710911.
Article6. Bobic S, van Drunen CM, Callebaut I, Hox V, Jorissen M, Fokkens WJ, et al. Dexamethasone-induced apoptosis of freshly isolated human nasal epithelial cells concomitant with abrogation of IL-8 production. Rhinology. 2010; 48(4):401–407. PMID: 21442075.
Article7. Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, et al. Estrogen receptors: how do they signal and what are their targets. Physiol Rev. 2007; 87(3):905–931. PMID: 17615392.
Article8. Philpott CM, Wild DC, Wolstensholme CR, Murty GE. The presence of ovarian hormone receptors in the nasal mucosa and their relationship to nasal symptoms. Rhinology. 2008; 46(3):221–225. PMID: 18853875.9. Nappi C, Di Spiezio Sardo A, Guerra G, Bifulco G, Testa D, Di Carlo C. Functional and morphologic evaluation of the nasal mucosa before and after hormone therapy in postmenopausal women with nasal symptoms. Fertil Steril. 2003; 80(3):669–671. PMID: 12969725.
Article10. Shirasaki H, Watanabe K, Kanaizumi E, Konno N, Sato J, Narita S, et al. Expression and localization of steroid receptors in human nasal mucosa. Acta Otolaryngol. 2004; 124(8):958–963. PMID: 15536653.
Article11. Millas I, Liquidato BM, Buck HS, Barros MD, Paes RA, Dolci JE. Evaluation of estrogenic receptors in the nasal mucosa of women taking oral contraceptives. Contraception. 2011; 83(6):571–577. PMID: 21570556.
Article12. Acconcia F, Totta P, Ogawa S, Cardillo I, Inoue S, Leone S, et al. Survival versus apoptotic 17beta-estradiol effect: role of ER alpha and ER beta activated non-genomic signaling. J Cell Physiol. 2005; 203(1):193–201. PMID: 15389627.13. Moorhead PS. Human tumor cell line with a quasi-diploid karyotype (RPMI 2650). Exp Cell Res. 1965; 39(1):190–196. PMID: 5831238.
Article14. Moll R, Krepler R, Franke WW. Complex cytokeratin polypeptide patterns observed in certain human carcinomas. Differentiation. 1983; 23(3):256–269. PMID: 6189757.
Article15. Zhao XJ, McKerr G, Dong Z, Higgins CA, Carson J, Yang ZQ, et al. Expression of oestrogen and progesterone receptors by mast cells alone, but not lymphocytes, macrophages or other immune cells in human upper airways. Thorax. 2001; 56(3):205–211. PMID: 11182013.
Article16. Yih WY, Richardson L, Kratochvil FJ, Avera SP, Zieper MB. Expression of estrogen receptors in desquamative gingivitis. J Periodontol. 2000; 71(3):482–487. PMID: 10776938.
Article17. Stabile LP, Davis AL, Gubish CT, Hopkins TM, Luketich JD, Christie N, et al. Human non-small cell lung tumors and cells derived from normal lung express both estrogen receptor alpha and beta and show biological responses to estrogen. Cancer Res. 2002; 62(7):2141–2150. PMID: 11929836.18. McDougall CM, Blaylock MG, Douglas JG, Brooker RJ, Helms PJ, Walsh GM. Nasal epithelial cells as surrogates for bronchial epithelial cells in airway inflammation studies. Am J Respir Cell Mol Biol. 2008; 39(5):560–568. PMID: 18483420.
Article19. Dorscheid DR, Wojcik KR, Sun S, Marroquin B, White SR. Apoptosis of airway epithelial cells induced by corticosteroids. Am J Respir Crit Care Med. 2001; 164(10 Pt 1):1939–1947. PMID: 11734450.
Article20. Harris RA, Hiles ID, Page MJ, O'Hare MJ. The induction of apoptosis in human mammary luminal epithelial cells by expression of activated c-neu and its abrogation by glucocorticoids. Br J Cancer. 1995; 72(2):386–392. PMID: 7640223.
Article21. Wen LP, Madani K, Fahrni JA, Duncan SR, Rosen GD. Dexamethasone inhibits lung epithelial cell apoptosis induced by IFN-gamma and Fas. Am J Physiol. 1997; 273(5):L921–L929. PMID: 9374718.22. Lee TH, Nam JG, Lee HM, Kim BY, Kang MK, Bae WY, et al. Dexamethasone induces apoptosis of nasal polyp-derived tissue cultures through JNK and p38 MAPK activation. Clin Exp Otorhinolaryngol. 2014; 7(2):112–118. PMID: 24917907.
Article23. Kamble PG, Pereira MJ, Almby K, Eriksson JW. Estrogen interacts with glucocorticoids in the regulation of lipocalin 2 expression in human adipose tissue. Reciprocal roles of estrogen receptor α and β in insulin resistance? Mol Cell Endocrinol. 2019; 490:28–36. PMID: 30953748.
Article24. Thornton MJ, Nelson LD, Taylor AH, Birch MP, Laing I, Messenger AG. The modulation of aromatase and estrogen receptor alpha in cultured human dermal papilla cells by dexamethasone: a novel mechanism for selective action of estrogen via estrogen receptor beta? J Invest Dermatol. 2006; 126(9):2010–2018. PMID: 16691199.25. Park JB. The effects of dexamethasone, ascorbic acid, and β-glycerophosphate on osteoblastic differentiation by regulating estrogen receptor and osteopontin expression. J Surg Res. 2012; 173(1):99–104. PMID: 21035140.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of Estrogen Receptor-alpha in the Regulation of Claudin-6 Expression in Breast Cancer Cells
- Bone Morphogenetic Protein-2 Desensitizes MC3T3-E1 Osteoblastic Cells to Estrogen Through Transcriptional Downregulation of Estrogen Receptor 1
- Expression of Cyclooxygenase-2 in Human Breast Carcinoma: Relevance to Tumor Angiogenesis and Expression of Estrogen Receptor
- Evidence for estrogen receptor expression during medullary bone formation and resorption in estrogen-treated male Japanese quails (Coturnix coturnix japonica)
- Study on the Role of Estrogen Receptor-Alpha in Yak-Kong and Soybean Induced Proliferation of MG-63 Human Osteoblastic Cells