Korean J Physiol Pharmacol.
2021 Jan;25(1):59-68. 10.4196/kjpp.2021.25.1.59.
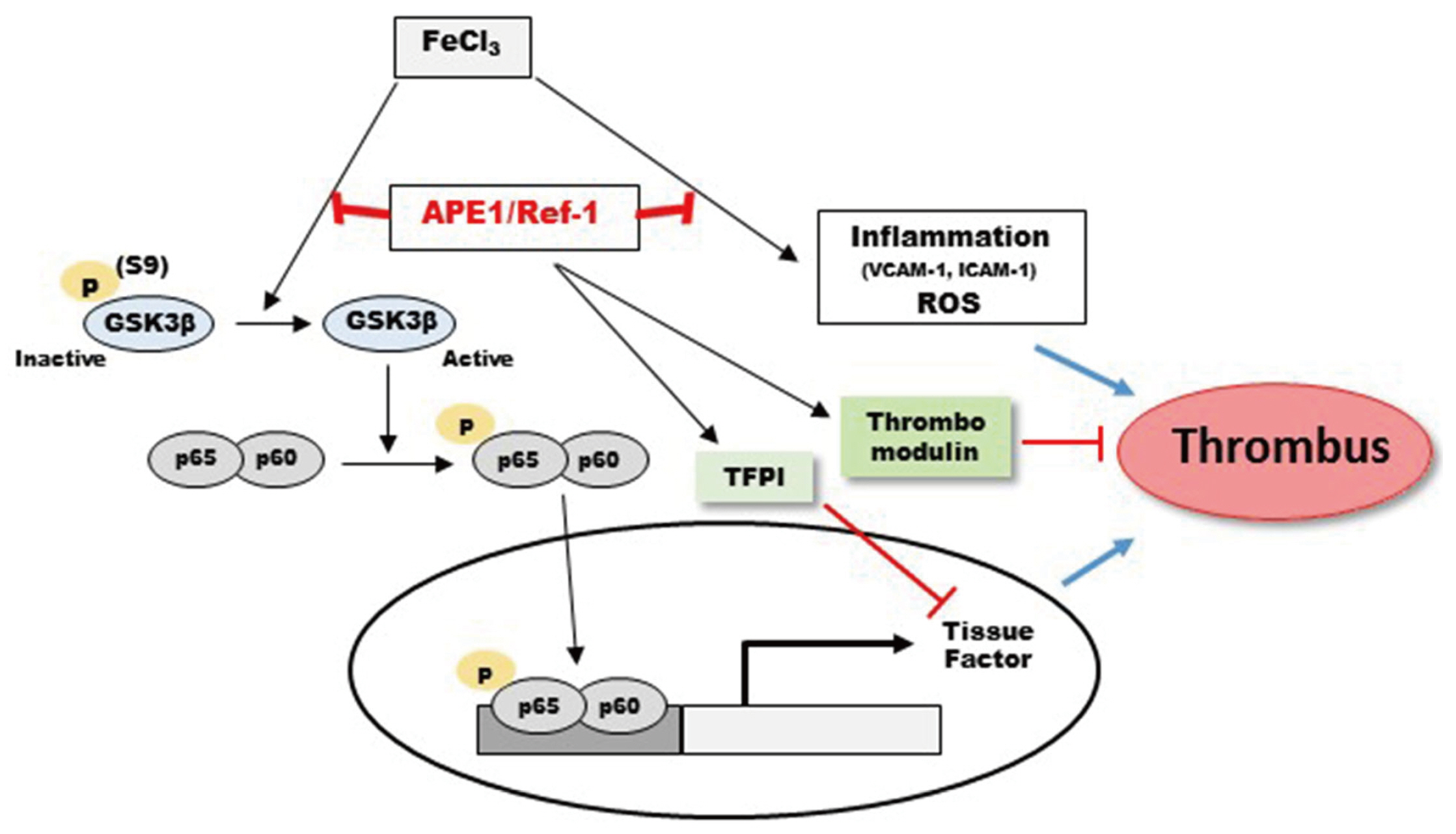
Ref-1 protects against FeCl3 -induced thrombosis and tissue factor expression via the GSK3β–NF-κB pathway
- Affiliations
-
- 1Departments of Physiology and Medical Science, Chungnam National University School of Medicine, Daejeon, Korea
- 2Departments of Surgery, Chungnam National University School of Medicine, Daejeon, Korea
- 3Departments of Plastic and Reconstructive Surgery, Chungnam National University School of Medicine, Daejeon, Korea
- 4Departments of Brain Research Institute, Chungnam National University School of Medicine, Daejeon, Korea
- 5Department of Thoracic and Cardiovascular Surgery, Chungnam National University Hospital, Chungnam National University School of Medicine, Daejeon 35015, Korea
- KMID: 2509655
- DOI: http://doi.org/10.4196/kjpp.2021.25.1.59
Abstract
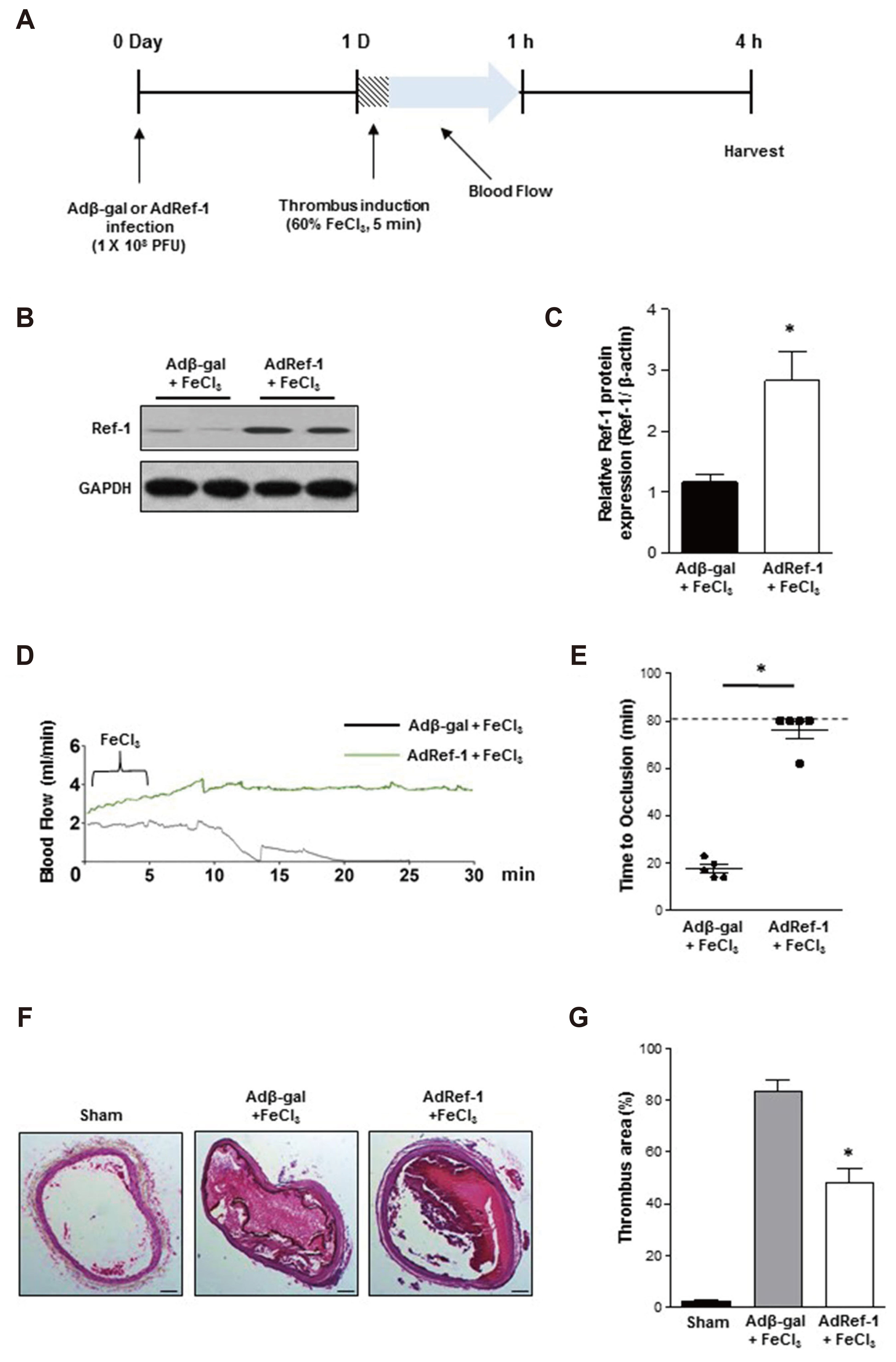
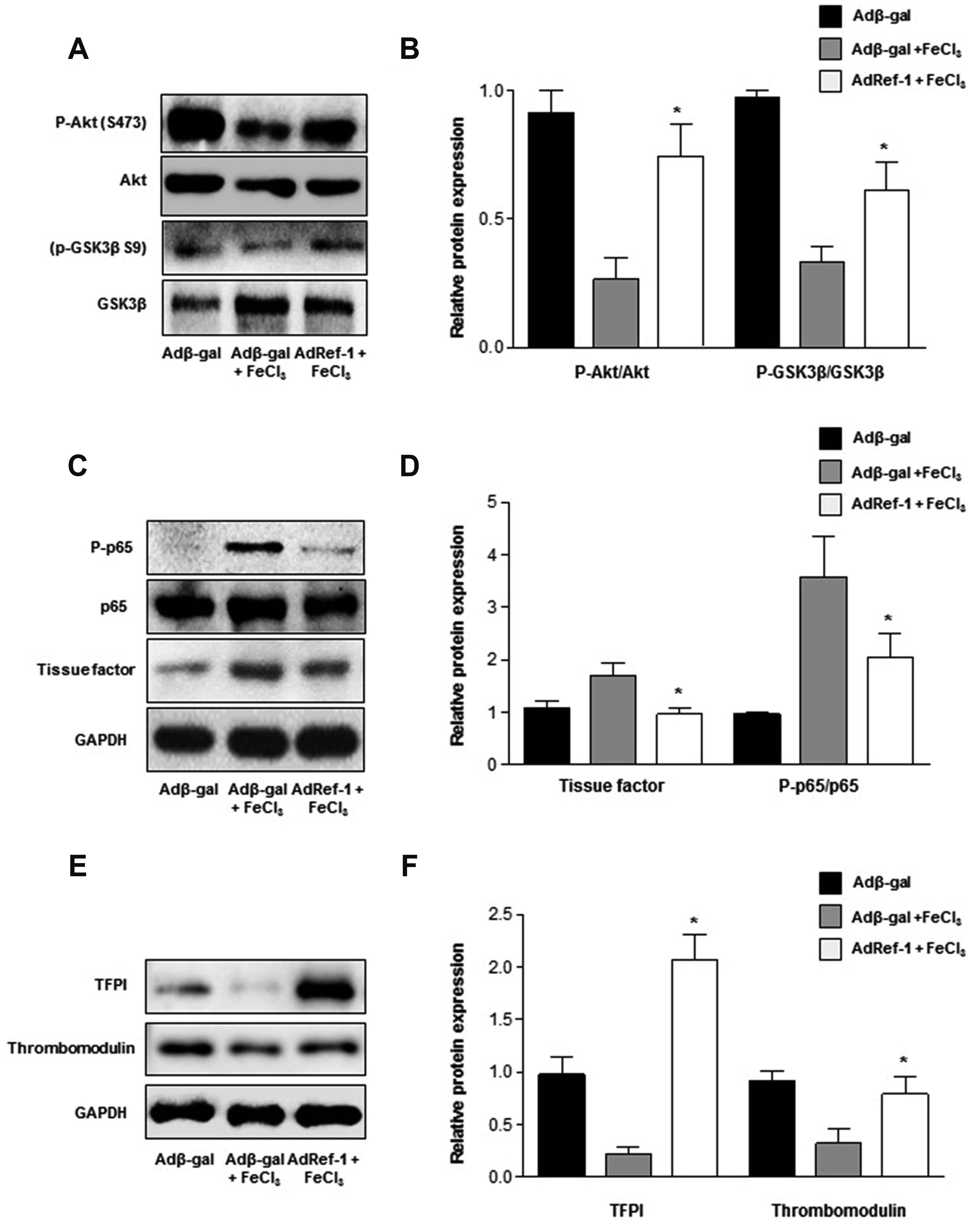
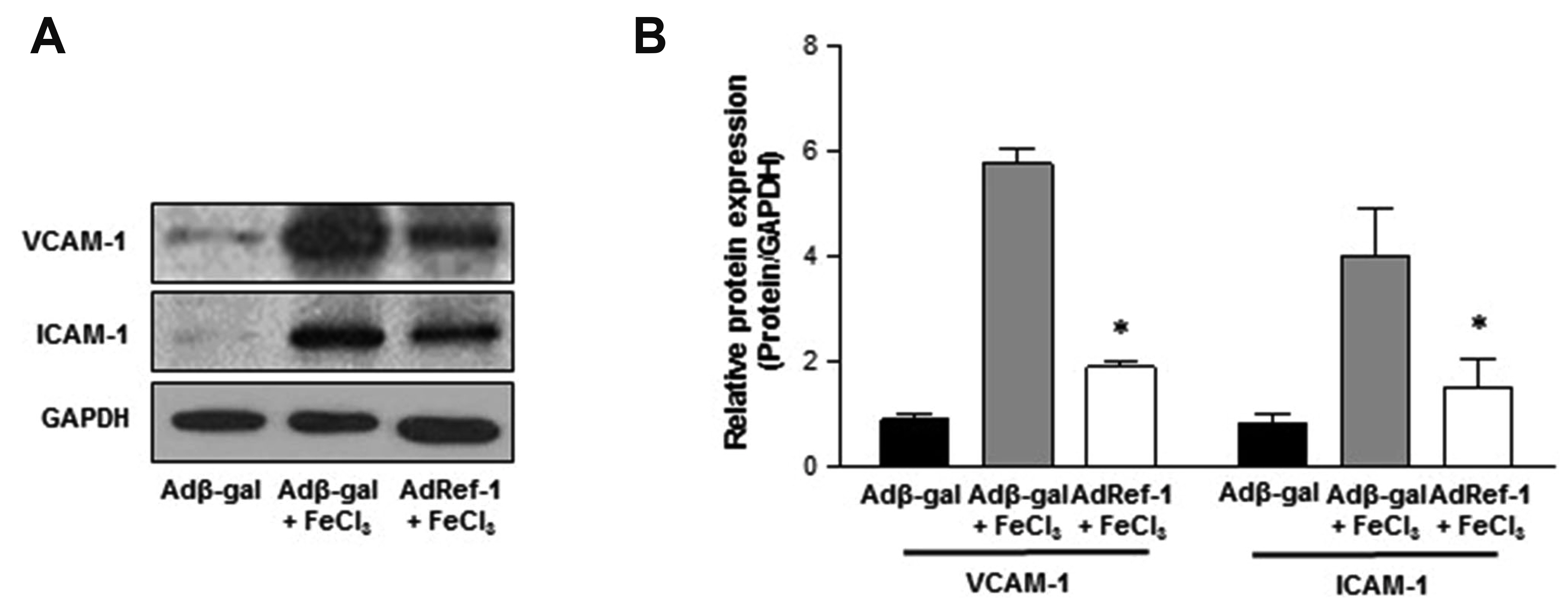
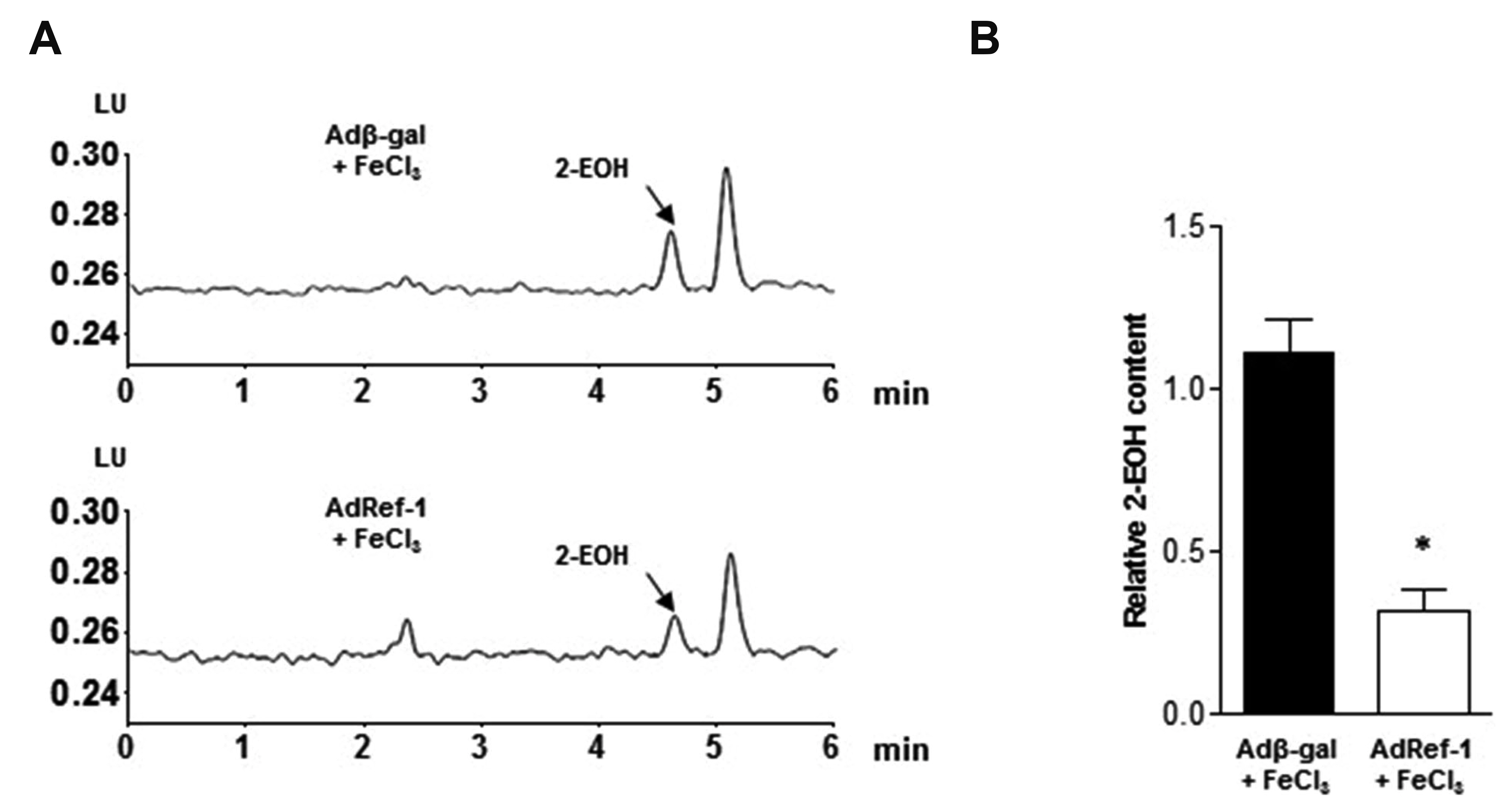
- Arterial thrombosis and its associated diseases are considered to constitute a major healthcare problem. Arterial thrombosis, defined as blood clot formation in an artery that interrupts blood circulation, is associated with many cardiovascular diseases. Oxidative stress is one of many important factors that aggravates the pathophysiological process of arterial thrombosis. Apurinic/apyrimidinic endonuclease 1/redox factor-1 (Ref-1) has a multifunctional role in cells that includes the regulation of oxidative stress and anti-inflammatory function. The aim of this study was to investigate the therapeutic effect of adenovirus-mediated Ref-1 overexpression on arterial thrombosis induced by 60% FeCl3 solution in rats. Blood flow was measured to detect the time to occlusion, thrombus formation was detected by hematoxylin and eosin staining, reactive oxygen species (ROS) levels were detected by high-performance liquid chromatography, and the expression of tissue factor and other proteins was detected by Western blot. FeCl3 aggravated thrombus formation in carotid arteries and reduced the time to artery occlusion. Ref-1 significantly delayed arterial obstruction via the inhibition of thrombus formation, especially by downregulating tissue factor expression through the Akt-GSK3β-NF-κB signaling pathway. Ref-1 also reduced the expression of vascular inflammation markers ICAM-1 and VCAM-1, and reduced the level of ROS that contributed to thrombus formation. The results showed that adenovirus-mediated Ref-1 overexpression reduced thrombus formation in the rat carotid artery. In summary, Ref-1 overexpression had anti-thrombotic effects in a carotid artery thrombosis model and could be a target for the treatment of arterial thrombosis.
Figure
Reference
-
1. Keenan NG, Sheppard MN, Nott DM, Pennell DJ, Mohiaddin RH. 2009; Carotid plaque rupture. Lancet. 374:1703. DOI: 10.1016/S0140-6736(09)60291-9. PMID: 19914514.
Article2. Blanco M, Sobrino T, Montaner J, Medrano V, Jiménez C, Masjuán J, Gómez-Escalonilla C, de Luis P, Arboix A, Castillo J. MITICO Study. 2010; Stroke with polyvascular atherothrombotic disease. Atherosclerosis. 208:587–592. DOI: 10.1016/j.atherosclerosis.2009.07.041. PMID: 19695570.
Article3. Santarius T, Menon DK. 2003; Images in clinical medicine. Carotid-artery thrombosis secondary to basal skull fracture. N Engl J Med. 349:e5. DOI: 10.1056/ENEJMicm020664. PMID: 12890856.4. Stoll G, Kleinschnitz C, Nieswandt B. 2008; Molecular mechanisms of thrombus formation in ischemic stroke: novel insights and targets for treatment. Blood. 112:3555–3562. DOI: 10.1182/blood-2008-04-144758. PMID: 18676880.
Article5. Zhang W, Wang J, Wang H, Tang R, Belcher JD, Viollet B, Geng JG, Zhang C, Wu C, Slungaard A, Zhu C, Huo Y. 2010; Acadesine inhibits tissue factor induction and thrombus formation by activating the phosphoinositide 3-kinase/Akt signaling pathway. Arterioscler Thromb Vasc Biol. 30:1000–1006. DOI: 10.1161/ATVBAHA.110.203141. PMID: 20185792. PMCID: PMC3626455.
Article6. Li YD, Ye BQ, Zheng SX, Wang JT, Wang JG, Chen M, Liu JG, Pei XH, Wang LJ, Lin ZX, Gupta K, Mackman N, Slungaard A, Key NS, Geng JG. 2009; NF-kappaB transcription factor p50 critically regulates tissue factor in deep vein thrombosis. J Biol Chem. 284:4473–4483. DOI: 10.1074/jbc.M806010200. PMID: 19095643. PMCID: PMC2640971.7. Johnson GJ, Leis LA, Bach RR. 2009; Tissue factor activity of blood mononuclear cells is increased after total knee arthroplasty. Thromb Haemost. 102:728–734. DOI: 10.1160/TH09-04-0261. PMID: 19806259.
Article8. Ruf W. 2012; Role of thiol pathways in TF procoagulant regulation. Thromb Res. 129 Suppl 2:S11–S12. DOI: 10.1016/j.thromres.2012.02.020. PMID: 22401798. PMCID: PMC3393083.
Article9. Evans CH. 1991; The role of proteinases in cartilage destruction. Agents Actions Suppl. 32:135–152. DOI: 10.1007/978-3-0348-7405-2_19. PMID: 2069082.
Article10. Li S, Chen H, Ren J, Geng Q, Song J, Lee C, Cao C, Zhang J, Xu N. 2014; MicroRNA-223 inhibits tissue factor expression in vascular endothelial cells. Atherosclerosis. 237:514–520. DOI: 10.1016/j.atherosclerosis.2014.09.033. PMID: 25463083.
Article11. Dong R, Chen W, Feng W, Xia C, Hu D, Zhang Y, Yang Y, Wang DW, Xu X, Tu L. 2015; Exogenous bradykinin inhibits tissue factor induction and deep vein thrombosis via activating the eNOS/phosphoinositide 3-kinase/Akt signaling pathway. Cell Physiol Biochem. 37:1592–1606. DOI: 10.1159/000438526. PMID: 26517864.
Article12. Schabbauer G, Tencati M, Pedersen B, Pawlinski R, Mackman N. 2004; PI3K-Akt pathway suppresses coagulation and inflammation in endotoxemic mice. Arterioscler Thromb Vasc Biol. 24:1963–1969. DOI: 10.1161/01.ATV.0000143096.15099.ce. PMID: 15319270.
Article13. Guha M, Mackman N. 2002; The phosphatidylinositol 3-kinase-Akt pathway limits lipopolysaccharide activation of signaling pathways and expression of inflammatory mediators in human monocytic cells. J Biol Chem. 277:32124–32132. DOI: 10.1074/jbc.M203298200. PMID: 12052830.
Article14. Winckers K, ten Cate H, Hackeng TM. 2013; The role of tissue factor pathway inhibitor in atherosclerosis and arterial thrombosis. Blood Rev. 27:119–132. DOI: 10.1016/j.blre.2013.03.001. PMID: 23631910.
Article15. Becker L, Prado K, Foppa M, Martinelli N, Aguiar C, Furian T, Clausell N, Rohde LE. 2012; Endothelial dysfunction assessed by brachial artery ultrasound in severe sepsis and septic shock. J Crit Care. 27:316.e9–316.e14. DOI: 10.1016/j.jcrc.2011.08.002. PMID: 22172795.
Article16. Karbach S, Wenzel P, Waisman A, Munzel T, Daiber A. 2014; eNOS uncoupling in cardiovascular diseases--the role of oxidative stress and inflammation. Curr Pharm Des. 20:3579–3594. DOI: 10.2174/13816128113196660748. PMID: 24180381.17. Steven S, Münzel T, Daiber A. 2015; Exploiting the pleiotropic antioxidant effects of established drugs in cardiovascular disease. Int J Mol Sci. 16:18185–18223. DOI: 10.3390/ijms160818185. PMID: 26251902. PMCID: PMC4581241.
Article18. Daiber A, Steven S, Weber A, Shuvaev VV, Muzykantov VR, Laher I, Li H, Lamas S, Münzel T. 2017; Targeting vascular (endothelial) dysfunction. Br J Pharmacol. 174:1591–1619. DOI: 10.1111/bph.13517. PMID: 27187006. PMCID: PMC5446575.
Article19. Steven S, Frenis K, Oelze M, Kalinovic S, Kuntic M, Bayo Jimenez MT, Vujacic-Mirski K, Helmstädter J, Kröller-Schön S, Münzel T, Daiber A. 2019; Vascular Inflammation and oxidative stress: major triggers for cardiovascular disease. Oxid Med Cell Longev. 2019:7092151. DOI: 10.1155/2019/7092151. PMID: 31341533. PMCID: PMC6612399.
Article20. Cimmino G, Cirillo P, Ragni M, Conte S, Uccello G, Golino P. 2015; Reactive oxygen species induce a procoagulant state in endothelial cells by inhibiting tissue factor pathway inhibitor. J Thromb Thrombolysis. 40:186–192. DOI: 10.1007/s11239-015-1199-1. PMID: 25712553.
Article21. Lee YR, Joo HK, Jeon BH. 2020; The biological role of apurinic/apyrimidinic endonuclease1/redox factor-1 as a therapeutic target for vascular inflammation and as a serologic biomarker. Biomedicines. 8:57. DOI: 10.3390/biomedicines8030057. PMID: 32164272. PMCID: PMC7148461.
Article22. Hao J, Du H, Liu F, Lu JC, Yang XC, Cui W. 2019; Apurinic/apyrimidinic endonuclease/redox factor 1 (APE1) alleviates myocardial hypoxia-reoxygenation injury by inhibiting oxidative stress and ameliorating mitochondrial dysfunction. Exp Ther Med. 17:2143–2151. DOI: 10.3892/etm.2019.7212. PMID: 30867702. PMCID: PMC6395998.
Article23. Park MS, Choi S, Lee YR, Joo HK, Kang G, Kim CS, Kim SJ, Lee SD, Jeon BH. 2016; Secreted APE1/Ref-1 inhibits TNF-α-stimulated endothelial inflammation via thiol-disulfide exchange in TNF receptor. Sci Rep. 6:23015. DOI: 10.1038/srep23015. PMID: 26964514. PMCID: PMC4786854.
Article24. Joo HK, Lee YR, Lee EO, Park MS, Choi S, Kim CS, Park JB, Jeon BH. 2019; The extracellular role of Ref-1 as anti-inflammatory function in lipopolysaccharide-induced septic mice. Free Radic Biol Med. 139:16–23. DOI: 10.1016/j.freeradbiomed.2019.05.013. PMID: 31100475.
Article25. Lee HM, Jeon BH, Won KJ, Lee CK, Park TK, Choi WS, Bae YM, Kim HS, Lee SK, Park SH, Irani K, Kim B. 2009; Gene transfer of redox factor-1 inhibits neointimal formation: involvement of platelet-derived growth factor-beta receptor signaling via the inhibition of the reactive oxygen species-mediated Syk pathway. Circ Res. 104:219–227. 5p following 227DOI: 10.1161/CIRCRESAHA.108.178699. PMID: 19038866.26. Libby P. 2002; Inflammation in atherosclerosis. Nature. 420:868–874. DOI: 10.1038/nature01323. PMID: 12490960.
Article27. Perlman H, Luo Z, Krasinski K, Le Roux A, Mahfoudi A, Smith RC, Branellec D, Walsh K. 1999; Adenovirus-mediated delivery of the Gax transcription factor to rat carotid arteries inhibits smooth muscle proliferation and induces apoptosis. Gene Ther. 6:758–763. DOI: 10.1038/sj.gt.3300893. PMID: 10505098.
Article28. Jeon BH, Gupta G, Park YC, Qi B, Haile A, Khanday FA, Liu YX, Kim JM, Ozaki M, White AR, Berkowitz DE, Irani K. 2004; Apurinic/apyrimidinic endonuclease 1 regulates endothelial NO production and vascular tone. Circ Res. 95:902–910. DOI: 10.1161/01.RES.0000146947.84294.4c. PMID: 15472121.29. Lee SK, Chung JI, Park MS, Joo HK, Lee EJ, Cho EJ, Park JB, Ryoo S, Irani K, Jeon BH. 2011; Apurinic/apyrimidinic endonuclease 1 inhibits protein kinase C-mediated p66shc phosphorylation and vasoconstriction. Cardiovasc Res. 91:502–509. DOI: 10.1093/cvr/cvr095. PMID: 21467074. PMCID: PMC3139447.
Article30. Kurz KD, Main BW, Sandusky GE. 1990; Rat model of arterial thrombosis induced by ferric chloride. Thromb Res. 60:269–280. DOI: 10.1016/0049-3848(90)90106-M. PMID: 2087688.
Article31. Robinson MA, Welsh DC, Bickel DJ, Lynch JJ, Lyle EA. 2003; Differential effects of sodium nitroprusside and hydralazine in a rat model of topical FeCl3-induced carotid artery thrombosis. Thromb Res. 111:59–64. DOI: 10.1016/j.thromres.2003.08.012. PMID: 14644081.32. Zielonka J, Hardy M, Kalyanaraman B. 2009; HPLC study of oxidation products of hydroethidine in chemical and biological systems: ramifications in superoxide measurements. Free Radic Biol Med. 46:329–338. DOI: 10.1016/j.freeradbiomed.2008.10.031. PMID: 19026738. PMCID: PMC3375818.
Article33. Di Santo A, Amore C, Dell'Elba G, Manarini S, Evangelista V. 2011; Glycogen synthase kinase-3 negatively regulates tissue factor expression in monocytes interacting with activated platelets. J Thromb Haemost. 9:1029–1039. DOI: 10.1111/j.1538-7836.2011.04236.x. PMID: 21320285. PMCID: PMC3091995.
Article34. Breitenstein A, Camici GG, Tanner FC. 2009; Tissue factor: beyond coagulation in the cardiovascular system. Clin Sci. 118:159–172. DOI: 10.1042/CS20080622. PMID: 19845509.
Article35. Eisenreich A, Malz R, Pepke W, Ayral Y, Poller W, Schultheiss HP, Rauch U. 2009; Role of the phosphatidylinositol 3-kinase/protein kinase B pathway in regulating alternative splicing of tissue factor mRNA in human endothelial cells. Circ J. 73:1746–1752. DOI: 10.1253/circj.CJ-99-0225. PMID: 19597299.
Article36. Risbud MV, Fertala J, Vresilovic EJ, Albert TJ, Shapiro IM. 2005; Nucleus pulposus cells upregulate PI3K/Akt and MEK/ERK signaling pathways under hypoxic conditions and resist apoptosis induced by serum withdrawal. Spine. 30:882–889. DOI: 10.1097/01.brs.0000159096.11248.6d. PMID: 15834331.
Article37. Olivares-Silva F, Landaeta R, Aránguiz P, Bolivar S, Humeres C, Anfossi R, Vivar R, Boza P, Muñoz C, Pardo-Jiménez V, Peiró C, Sánchez-Ferrer CF, Díaz-Araya G. 2018; Heparan sulfate potentiates leukocyte adhesion on cardiac fibroblast by enhancing Vcam-1 and Icam-1 expression. Biochim Biophys Acta Mol Basis Dis. 1864:831–842. DOI: 10.1016/j.bbadis.2017.12.002. PMID: 29222072.
Article38. Zibara K, Chignier E, Covacho C, Poston R, Canard G, Hardy P, McGregor J. 2000; Modulation of expression of endothelial intercellular adhesion molecule-1, platelet-endothelial cell adhesion molecule-1, and vascular cell adhesion molecule-1 in aortic arch lesions of apolipoprotein E-deficient compared with wild-type mice. Arterioscler Thromb Vasc Biol. 20:2288–2296. DOI: 10.1161/01.ATV.20.10.2288. PMID: 11031217.
Article39. Griendling KK, FitzGerald GA. 2003; Oxidative stress and cardiovascular injury: part II: animal and human studies. Circulation. 108:2034–2040. DOI: 10.1161/01.CIR.0000093661.90582.c4. PMID: 14581381.40. Sies H. 2015; Oxidative stress: a concept in redox biology and medicine. Redox Biol. 4:180–183. DOI: 10.1016/j.redox.2015.01.002. PMID: 25588755. PMCID: PMC4309861.
Article41. Qiao J, Arthur JF, Gardiner EE, Andrews RK, Zeng L, Xu K. 2018; Regulation of platelet activation and thrombus formation by reactive oxygen species. Redox Biol. 14:126–130. DOI: 10.1016/j.redox.2017.08.021. PMID: 28888895. PMCID: PMC5596263.
Article42. Furie B, Furie BC. 2008; Mechanisms of thrombus formation. N Engl J Med. 359:938–949. DOI: 10.1056/NEJMra0801082. PMID: 18753650.
Article43. Lu J, Xiang G, Liu M, Mei W, Xiang L, Dong J. 2015; Irisin protects against endothelial injury and ameliorates atherosclerosis in apolipoprotein E-Null diabetic mice. Atherosclerosis. 243:438–448. DOI: 10.1016/j.atherosclerosis.2015.10.020. PMID: 26520898.
Article44. Yau JW, Teoh H, Verma S. 2015; Endothelial cell control of thrombosis. BMC Cardiovasc Disord. 15:130. DOI: 10.1186/s12872-015-0124-z. PMID: 26481314. PMCID: PMC4617895.
Article45. Ren M, Li R, Luo M, Chen N, Deng X, Yan K, Zeng M, Wu J. 2014; Endothelial cells but not platelets are the major source of Toll-like receptor 4 in the arterial thrombosis and tissue factor expression in mice. Am J Physiol Regul Integr Comp Physiol. 307:R901–R907. DOI: 10.1152/ajpregu.00324.2014. PMID: 25275013.
Article46. Neeves KB. 2015; Physiochemical artifacts in FeCl3 thrombosis models. Blood. 126:700–701. DOI: 10.1182/blood-2015-05-644708. PMID: 26251225.47. Angkeow P, Deshpande SS, Qi B, Liu YX, Park YC, Jeon BH, Ozaki M, Irani K. 2002; Redox factor-1: an extra-nuclear role in the regulation of endothelial oxidative stress and apoptosis. Cell Death Differ. 9:717–725. DOI: 10.1038/sj.cdd.4401025. PMID: 12058277.
Article48. Ozaki M, Suzuki S, Irani K. 2002; Redox factor-1/APE suppresses oxidative stress by inhibiting the rac1 GTPase. FASEB J. 16:889–890. DOI: 10.1096/fj.01-0664fje. PMID: 12039869.49. Guo Y, Chen J, Zhao T, Fan Z. 2008; Granzyme K degrades the redox/DNA repair enzyme Ape1 to trigger oxidative stress of target cells leading to cytotoxicity. Mol Immunol. 45:2225–2235. DOI: 10.1016/j.molimm.2007.11.020. PMID: 18179823.
Article50. Kim CS, Son SJ, Kim EK, Kim SN, Yoo DG, Kim HS, Ryoo SW, Lee SD, Irani K, Jeon BH. 2006; Apurinic/apyrimidinic endonuclease1/redox factor-1 inhibits monocyte adhesion in endothelial cells. Cardiovasc Res. 69:520–526. DOI: 10.1016/j.cardiores.2005.10.014. PMID: 16325162.
Article51. Hall JL, Wang X, Van Adamson , Zhao Y, Gibbons GH. 2001; Overexpression of Ref-1 inhibits hypoxia and tumor necrosis factor-induced endothelial cell apoptosis through nuclear factor-kappab-independent and -dependent pathways. Circ Res. 88:1247–1253. DOI: 10.1161/hh1201.091796. PMID: 11420300.52. Yuk JM, Yang CS, Shin DM, Kim KK, Lee SK, Song YJ, Lee HM, Cho CH, Jeon BH, Jo EK. 2009; A dual regulatory role of apurinic/apyrimidinic endonuclease 1/redox factor-1 in HMGB1-induced inflammatory responses. Antioxid Redox Signal. 11:575–588. DOI: 10.1089/ars.2008.2196. PMID: 18715145.
Article53. Zhang WJ, Wei H, Hagen T, Frei B. 2007; Alpha-lipoic acid attenuates LPS-induced inflammatory responses by activating the phosphoinositide 3-kinase/Akt signaling pathway. Proc Natl Acad Sci U S A. 104:4077–4082. DOI: 10.1073/pnas.0700305104. PMID: 17360480. PMCID: PMC1805485.54. Yu M, Ge C, Zeng W, Mi Y, Zhang C. 2012; Retinoic acid promotes proliferation of chicken primordial germ cells via activation of PI3K/Akt-mediated NF-κB signalling cascade. Cell Biol Int. 36:705–712. DOI: 10.1042/CBI20110542. PMID: 22548360.
Article55. Sorensen BB, Rao LV, Tornehave D, Gammeltoft S, Petersen LC. 2003; Antiapoptotic effect of coagulation factor VIIa. Blood. 102:1708–1715. DOI: 10.1182/blood-2003-01-0157. PMID: 12738672.
Article56. Mast AE. 2016; Tissue factor pathway inhibitor: multiple anticoagulant activities for a single protein. Arterioscler Thromb Vasc Biol. 36:9–14. DOI: 10.1161/ATVBAHA.115.305996. PMID: 26603155. PMCID: PMC4690769.57. Maeda NY, Clavé MM, Bydlowski SP, Lopes AA. 2016; Decreased circulating thrombomodulin is improved by tadalafil therapy in hypoxemic patients with advanced pulmonary arterial hypertension. Thromb Res. 146:15–19. DOI: 10.1016/j.thromres.2016.08.016. PMID: 27564658.
Article58. Cybulsky MI, Gimbrone MA Jr. 1991; Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science. 251:788–791. DOI: 10.1126/science.1990440. PMID: 1990440.
Article59. Ambrosio G, Tritto I, Chiariello M. 1995; The role of oxygen free radicals in preconditioning. J Mol Cell Cardiol. 27:1035–1039. DOI: 10.1016/0022-2828(95)90072-1. PMID: 7563100.
Article60. Golino P, Ragni M, Cirillo P, Avvedimento VE, Feliciello A, Esposito N, Scognamiglio A, Trimarco B, Iaccarino G, Condorelli M, Chiariello M, Ambrosio G. 1996; Effects of tissue factor induced by oxygen free radicals on coronary flow during reperfusion. Nat Med. 2:35–40. DOI: 10.1038/nm0196-35. PMID: 8564835.
Article61. Tritto I, D'Andrea D, Eramo N, Scognamiglio A, De Simone C, Violante A, Esposito A, Chiariello M, Ambrosio G. 1997; Oxygen radicals can induce preconditioning in rabbit hearts. Circ Res. 80:743–748. DOI: 10.1161/01.RES.80.5.743. PMID: 9130455.
Article62. Tritto I, Duilio C, Santoro G, Elia PP, Cirillo P, De Simone C, Chiariello M, Ambrosio G. 1998; A short burst of oxygen radicals at reflow induces sustained release of oxidized glutathione from postischemic hearts. Free Radic Biol Med. 24:290–297. DOI: 10.1016/S0891-5849(97)00229-3. PMID: 9433904.
Article63. Khechai F, Ollivier V, Bridey F, Amar M, Hakim J, de Prost D. 1997; Effect of advanced glycation end product-modified albumin on tissue factor expression by monocytes. Role of oxidant stress and protein tyrosine kinase activation. Arterioscler Thromb Vasc Biol. 17:2885–2890. DOI: 10.1161/01.ATV.17.11.2885. PMID: 9409271.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Eriodictyol Inhibits the Production and Gene Expression of MUC5AC Mucin via the IκBα-NF-κB p65 Signaling Pathway in Airway Epithelial Cells
- Wnt-C59 inhibits proinflammatory cytokine expression by reducing the interaction between β-catenin and NF-κB in LPS-stimulated epithelial and macrophage cells
- Porcine parvovirus nonstructural protein NS1 activates NF-κB and it involves TLR2 signaling pathway
- Kaempferol Regulates the Expression of Airway MUC5AC Mucin Gene via IκBα-NF-κB p65 and p38-p44/42-Sp1 Signaling Pathways
- Activation of CpG-ODN-Induced TLR9 Signaling Inhibited by Interleukin-37 in U937 Human Macrophages