Korean J Physiol Pharmacol.
2021 Jan;25(1):27-38. 10.4196/kjpp.2021.25.1.27.
Gamma-aminobutyric acid-salt attenuated high cholesterol/high salt diet induced hypertension in mice
- Affiliations
-
- 1Department of Anatomy and Cell Biology, Gachon University College of Medicine, Korea
- 2Functional Cellular Networks Laboratory, Lee Gil Ya Cancer and Diabetes Institute, Gachon University, Incheon 21999, Korea
- 3Marine Bioprocess Co., Ltd., Busan 46048, Korea
- 4Department of Thoracic and Cardiovascular Surgery, Gachon University Gil Medical Center, Gachon University, Incheon 21565, Korea
- KMID: 2509652
- DOI: http://doi.org/10.4196/kjpp.2021.25.1.27
Abstract
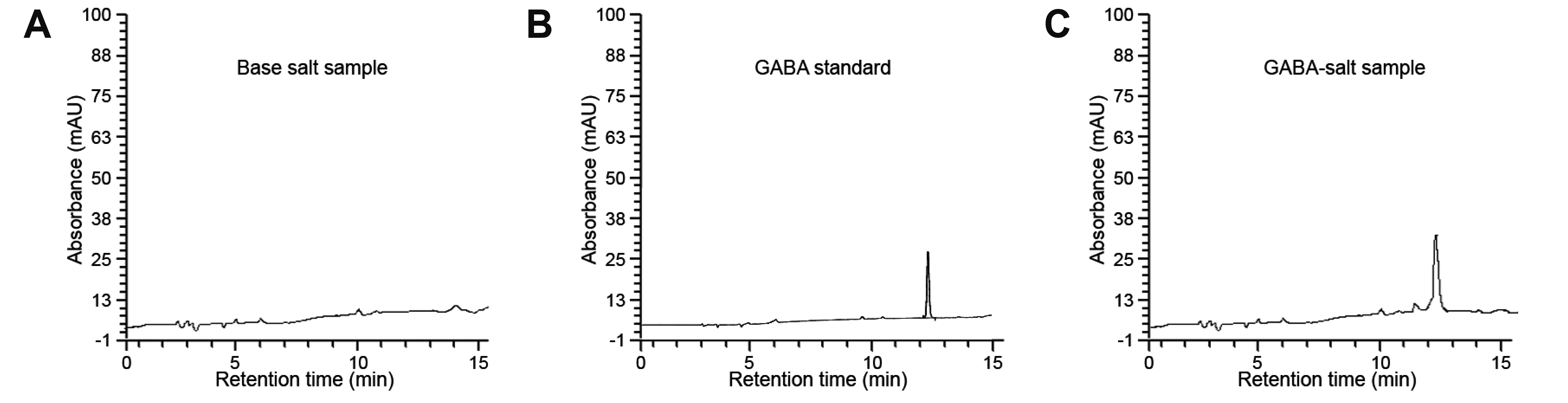
- Excessive salt intake induces hypertension, but several gamma-aminobutyric acid (GABA) supplements have been shown to reduce blood pressure. GABAsalt, a fermented salt by L. brevis BJ20 containing GABA was prepared through the post-fermentation with refined salt and the fermented GABA extract. We evaluated the effect of GABA-salt on hypertension in a high salt, high cholesterol diet induced mouse model. We analyzed type 1 macrophage (M1) polarization, the expression of M1 related cytokines, GABA receptor expression, endothelial cell (EC) dysfunction, vascular smooth muscle cell (VSMC) proliferation, and medial thicknesses in mice model. GABA-salt attenuated diet-induced blood pressure increases, M1 polarization, and TNF-α and inducible nitric oxide synthase (NOS) levels in mouse aortas, and in salt treated macrophages in vitro. Furthermore, GABA-salt induced higher GABAB receptor and endothelial NOS (eNOS) and eNOS phosphorylation levels than those observed in salt treated ECs. In addition, GABA-salt attenuated EC dysfunction by decreasing the levels of adhesion molecules (E-selectin, Intercellular Adhesion Molecule-1 [ICAM-1], vascular cell adhesion molecule-1 [VCAM-1]) and of von Willebrand Factor and reduced EC death. GABA-salt also reduced diet-induced reductions in the levels of eNOS, phosphorylated eNOS, VSMC proliferation and medial thickening in mouse aortic tissues, and attenuated Endothelin-1 levels in salt treated VSMCs. In summary, GABA-salt reduced high salt, high cholesterol diet induced hypertension in our mouse model by reducing M1 polarization, EC dysfunction, and VSMC proliferation.
Keyword
Figure
Reference
-
1. World Health Organization (WHO). 2009. Global health risks: mortality and burden of disease attributable to selected major risks. World Health Organization;Geneva:2. Liu G, Hitomi H, Rahman A, Nakano D, Mori H, Masaki T, Ma H, Iwamoto T, Kobori H, Nishiyama A. 2014; High sodium augments angiotensin II-induced vascular smooth muscle cell proliferation through the ERK 1/2-dependent pathway. Hypertens Res. 37:13–18. DOI: 10.1038/hr.2013.108. PMID: 24026042. PMCID: PMC3947370.
Article3. Manning RD Jr, Hu L, Tan DY, Meng S. 2001; Role of abnormal nitric oxide systems in salt-sensitive hypertension. Am J Hypertens. 14(6 Pt 2):68S–73S. DOI: 10.1016/S0895-7061(01)02072-6. PMID: 11411768.
Article4. Chiong M, Morales P, Torres G, Gutiérrez T, García L, Ibacache M, Michea L. 2013; Influence of glucose metabolism on vascular smooth muscle cell proliferation. Vasa. 42:8–16. DOI: 10.1024/0301-1526/a000243. PMID: 23385222.
Article5. Liu Y, Rusch NJ, Lombard JH. 1999; Loss of endothelium and receptor-mediated dilation in pial arterioles of rats fed a short-term high salt diet. Hypertension. 33:686–688. DOI: 10.1161/01.HYP.33.2.686. PMID: 10024328.
Article6. Frisbee JC, Sylvester FA, Lombard JH. 2001; High-salt diet impairs hypoxia-induced cAMP production and hyperpolarization in rat skeletal muscle arteries. Am J Physiol Heart Circ Physiol. 281:H1808–H1815. DOI: 10.1152/ajpheart.2001.281.4.H1808. PMID: 11557575.
Article7. Liu Y, Fredricks KT, Roman RJ, Lombard JH. 1997; Response of resistance arteries to reduced PO2 and vasodilators during hypertension and elevated salt intake. Am J Physiol. 273(2 Pt 2):H869–H877. DOI: 10.1152/ajpheart.1997.273.2.H869. PMID: 9277505.
Article8. Parissis JT, Korovesis S, Giazitzoglou E, Kalivas P, Katritsis D. 2002; Plasma profiles of peripheral monocyte-related inflammatory markers in patients with arterial hypertension. Correlations with plasma endothelin-1. Int J Cardiol. 83:13–21. DOI: 10.1016/S0167-5273(02)00021-9. PMID: 11959378.
Article9. Justin Rucker A, Crowley SD. 2017; The role of macrophages in hypertension and its complications. Pflugers Arch. 469:419–430. DOI: 10.1007/s00424-017-1950-x. PMID: 28251313. PMCID: PMC5773253.
Article10. Mirhafez SR, Mohebati M, Feiz Disfani M, Saberi Karimian M, Ebrahimi M, Avan A, Eslami S, Pasdar A, Rooki H, Esmaeili H, Ferns GA, Ghayour-Mobarhan M. 2014; An imbalance in serum concentrations of inflammatory and anti-inflammatory cytokines in hypertension. J Am Soc Hypertens. 8:614–623. DOI: 10.1016/j.jash.2014.05.007. PMID: 25224864.
Article11. Harwani SC. 2018; Macrophages under pressure: the role of macrophage polarization in hypertension. Transl Res. 191:45–63. DOI: 10.1016/j.trsl.2017.10.011. PMID: 29172035. PMCID: PMC5733698.
Article12. Martinez FO, Helming L, Gordon S. 2009; Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 27:451–483. DOI: 10.1146/annurev.immunol.021908.132532. PMID: 19105661.
Article13. Sica A, Mantovani A. 2012; Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 122:787–795. DOI: 10.1172/JCI59643. PMID: 22378047. PMCID: PMC3287223.
Article14. Lu G, Zhang R, Geng S, Peng L, Jayaraman P, Chen C, Xu F, Yang J, Li Q, Zheng H, Shen K, Wang J, Liu X, Wang W, Zheng Z, Qi CF, Si C, He JC, Liu K, Lira SA, et al. 2015; Myeloid cell-derived inducible nitric oxide synthase suppresses M1 macrophage polarization. Nat Commun. 6:6676. DOI: 10.1038/ncomms7676. PMID: 25813085. PMCID: PMC4389243.
Article15. Gordon S, Martinez FO. 2010; Alternative activation of macrophages: mechanism and functions. Immunity. 32:593–604. DOI: 10.1016/j.immuni.2010.05.007. PMID: 20510870.
Article16. Hucke S, Eschborn M, Liebmann M, Herold M, Freise N, Engbers A, Ehling P, Meuth SG, Roth J, Kuhlmann T, Wiendl H, Klotz L. 2016; Sodium chloride promotes pro-inflammatory macrophage polarization thereby aggravating CNS autoimmunity. J Autoimmun. 67:90–101. DOI: 10.1016/j.jaut.2015.11.001. PMID: 26584738.
Article17. Inoue K, Shirai T, Ochiai H, Kasao M, Hayakawa K, Kimura M, Sansawa H. 2003; Blood-pressure-lowering effect of a novel fermented milk containing gamma-aminobutyric acid (GABA) in mild hypertensives. Eur J Clin Nutr. 57:490–495. DOI: 10.1038/sj.ejcn.1601555. PMID: 12627188.18. Hayakawa K, Kimura M, Kasaha K, Matsumoto K, Sansawa H, Yamori Y. 2004; Effect of a gamma-aminobutyric acid-enriched dairy product on the blood pressure of spontaneously hypertensive and normotensive Wistar-Kyoto rats. Br J Nutr. 92:411–417. DOI: 10.1079/BJN20041221. PMID: 15469644.19. Nishimura M, Yoshida S, Haramoto M, Mizuno H, Fukuda T, Kagami-Katsuyama H, Tanaka A, Ohkawara T, Sato Y, Nishihira J. 2015; Effects of white rice containing enriched gamma-aminobutyric acid on blood pressure. J Tradit Complement Med. 6:66–71. DOI: 10.1016/j.jtcme.2014.11.022. PMID: 26870683. PMCID: PMC4738072.
Article20. Tanaka H, Watanabe K, Ma M, Hirayama M, Kobayashi T, Oyama H, Sakaguchi Y, Kanda M, Kodama M, Aizawa Y. 2009; The effects of gamma-aminobutyric acid, vinegar, and dried bonito on blood pressure in normotensive and mildly or moderately hypertensive volunteers. J Clin Biochem Nutr. 45:93–100. DOI: 10.3164/jcbn.09-04. PMID: 19590713. PMCID: PMC2704332.21. Inoue K, Shirai T, Ochiai H, Kasao M, Hayakawa K, Kimura M, Sansawa H. 2003; Blood-pressure-lowering effect of a novel fermented milk containing gamma-aminobutyric acid (GABA) in mild hypertensives. Eur J Clin Nutr. 57:490–495. DOI: 10.1038/sj.ejcn.1601555. PMID: 12627188.22. Matsubara F, Ueno H, Tadano K, Suyama T, Imaizumi K, Suzuki T, Magata K, Kikuchi N, Muneyuki K, Nakamichi N, Kumagai H, Saruta T. 2002; Effects of GABA supplementation on blood pressure and safety in adults with mild hypertension. Jpn Pharmacol Ther. 30:963–972.23. Weinberger MH, Fineberg NS. 1991; Sodium and volume sensitivity of blood pressure. Age and pressure change over time. Hypertension. 18:67–71. DOI: 10.1161/01.HYP.18.1.67. PMID: 1860713.
Article24. Elijovich F, Weinberger MH, Anderson CA, Appel LJ, Bursztyn M, Cook NR, Dart RA, Newton-Cheh CH, Sacks FM, Laffer CL. 2016; Salt sensitivity of blood pressure: a scientific statement from the American Heart Association. Hypertension. 68:e7–e46. DOI: 10.1161/HYP.0000000000000047. PMCID: PMC4900938. PMID: 27443572.25. BJ Lee HS Song YS Kim MY Kim SJ Kwon . Marine Bioprocess Co., Ltd.2017. Jul. 20. Fermented salt containing GABA and preparing method thereof. Korea patent 10-1761710.26. BJ Lee HS Song YS Kim MY Kim SJ Kwon . Marine Bioprocess Co., Ltd.2017. Jul. 20. Low salinity fermented salt containing GABA and preparing method thereof. Korea patent 10-1761711.27. Bae JS, Oh AR, Lee HJ, Ahn YH, Cha JY. 2016; Hepatic Elovl6 gene expression is regulated by the synergistic action of ChREBP and SREBP-1c. Biochem Biophys Res Commun. 478:1060–1066. DOI: 10.1016/j.bbrc.2016.08.061. PMID: 27524233.
Article28. Kim OH, Booth CJ, Choi HS, Lee J, Kang J, Hur J, Jung WJ, Jung YS, Choi HJ, Kim H, Auh JH, Kim JW, Cha JY, Lee YJ, Lee CS, Choi C, Jung YJ, Yang JY, Im SS, Lee DH, et al. 2020; High-phytate/low-calcium diet is a risk factor for crystal nephropathies, renal phosphate wasting, and bone loss. Elife. 9:e52709. DOI: 10.7554/eLife.52709. PMID: 32271147. PMCID: PMC7145417.
Article29. Lee EH, Itan M, Jang J, Gu HJ, Rozenberg P, Mingler MK, Wen T, Yoon J, Park SY, Roh JY, Choi CS, Park WJ, Munitz A, Jung Y. 2018; Eosinophils support adipocyte maturation and promote glucose tolerance in obesity. Sci Rep. 8:9894. DOI: 10.1038/s41598-018-28371-4. PMID: 29967467. PMCID: PMC6028436.
Article30. Denton D, Weisinger R, Mundy NI, Wickings EJ, Dixson A, Moisson P, Pingard AM, Shade R, Carey D, Ardaillou R, Paillard F, Chapman J, Thillet J, Michel JB. 1995; The effect of increased salt intake on blood pressure of chimpanzees. Nat Med. 1:1009–1016. DOI: 10.1038/nm1095-1009. PMID: 7489355.
Article31. Elliott P, Walker LL, Little MP, Blair-West JR, Shade RE, Lee DR, Rouquet P, Leroy E, Jeunemaitre X, Ardaillou R, Paillard F, Meneton P, Denton DA. 2007; Change in salt intake affects blood pressure of chimpanzees: implications for human populations. Circulation. 116:1563–1568. DOI: 10.1161/CIRCULATIONAHA.106.675579. PMID: 17785625.32. Liu Y, Li H, Hong S, Yin X. 2015; Salt reduction and hypertension in China: a concise state-of-the-art review. Cardiovasc Diagn Ther. 5:191–196. DOI: 10.3978/j.issn.2223-3652.2015.05.01. PMID: 26090330. PMCID: PMC4451313.33. Brown IJ, Tzoulaki I, Candeias V, Elliott P. 2009; Salt intakes around the world: implications for public health. Int J Epidemiol. 38:791–813. DOI: 10.1093/ije/dyp139. PMID: 19351697.
Article34. Schatz V, Neubert P, Schröder A, Binger K, Gebhard M, Müller DN, Luft FC, Titze J, Jantsch J. 2017; Elementary immunology: Na+ as a regulator of immunity. Pediatr Nephrol. 32:201–210. DOI: 10.1007/s00467-016-3349-x. PMID: 26921211. PMCID: PMC5203836.35. Kostyk AG, Dahl KM, Wynes MW, Whittaker LA, Weiss DJ, Loi R, Riches DW. 2006; Regulation of chemokine expression by NaCl occurs independently of cystic fibrosis transmembrane conductance regulator in macrophages. Am J Pathol. 169:12–20. DOI: 10.2353/ajpath.2006.051042. PMID: 16816357. PMCID: PMC1698750.
Article36. Pfeffer K, Matsuyama T, Kündig TM, Wakeham A, Kishihara K, Shahinian A, Wiegmann K, Ohashi PS, Krönke M, Mak TW. 1993; Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 73:457–467. DOI: 10.1016/0092-8674(93)90134-C. PMID: 8387893.
Article37. Oliveira-Paula GH, Lacchini R, Tanus-Santos JE. 2014; Inducible nitric oxide synthase as a possible target in hypertension. Curr Drug Targets. 15:164–174. DOI: 10.2174/13894501113146660227. PMID: 24102471.
Article38. Deanfield JE, Halcox JP, Rabelink TJ. 2007; Endothelial function and dysfunction: testing and clinical relevance. Circulation. 115:1285–1295. DOI: 10.1161/CIRCULATIONAHA.106.652859. PMID: 17353456.39. Rainger GE, Nash GB. 2001; Cellular pathology of atherosclerosis: smooth muscle cells prime cocultured endothelial cells for enhanced leukocyte adhesion. Circ Res. 88:615–622. DOI: 10.1161/01.RES.88.6.615. PMID: 11282896.40. Chen CN, Chang SF, Lee PL, Chang K, Chen LJ, Usami S, Chien S, Chiu JJ. 2006; Neutrophils, lymphocytes, and monocytes exhibit diverse behaviors in transendothelial and subendothelial migrations under coculture with smooth muscle cells in disturbed flow. Blood. 107:1933–1942. DOI: 10.1182/blood-2005-08-3137. PMID: 16293605. PMCID: PMC1895706.
Article41. Vanhoutte PM. 2001; Endothelium-derived free radicals: for worse and for better. J Clin Invest. 107:23–25. DOI: 10.1172/JCI11832. PMID: 11134174. PMCID: PMC198553.
Article42. ivastava K Sr, Chandra S, Narang R, Bhatia J, Saluja D. 2018; E-selectin gene in essential hypertension: a case-control study. Eur J Clin Invest. 48:e12868. DOI: 10.1111/eci.12868. PMID: 29178542.
Article43. Harwani SC, Ratcliff J, Sutterwala FS, Ballas ZK, Meyerholz DK, Chapleau MW, Abboud FM. 2016; Nicotine mediates CD161a+ renal macrophage infiltration and premature hypertension in the spontaneously hypertensive rat. Circ Res. 119:1101–1115. DOI: 10.1161/CIRCRESAHA.116.309402. PMID: 27660287. PMCID: PMC5085865.44. Lin CH, Lee SY, Zhang CC, Du YF, Hung HC, Wu HT, Ou HY. 2016; Fenretinide inhibits macrophage inflammatory mediators and controls hypertension in spontaneously hypertensive rats via the peroxisome proliferator-activated receptor gamma pathway. Drug Des Devel Ther. 10:3591–3597. DOI: 10.2147/DDDT.S114879. PMID: 27843299. PMCID: PMC5098527.
Article45. Durán WN, Breslin JW, Sánchez FA. 2010; The NO cascade, eNOS location, and microvascular permeability. Cardiovasc Res. 87:254–261. DOI: 10.1093/cvr/cvq139. PMID: 20462865. PMCID: PMC2895543.46. Prabhakar P, Thatte HS, Goetz RM, Cho MR, Golan DE, Michel T. 1998; Receptor-regulated translocation of endothelial nitric-oxide synthase. J Biol Chem. 273:27383–27388. DOI: 10.1074/jbc.273.42.27383. PMID: 9765266.
Article47. Wang XP, Cheng ZY, Schmid KL. 2014; GABAB receptors expressed in human aortic endothelial cells mediate intracellular calcium concentration regulation and endothelial nitric oxide synthase translocation. Biomed Res Int. 2014:871735. DOI: 10.1155/2014/871735. PMID: 25114926. PMCID: PMC4119922.48. Hinds K, Monaghan KP, Frølund B, McGeown JG, Curtis TM. 2013; GABAergic control of arteriolar diameter in the rat retina. Invest Ophthalmol Vis Sci. 54:6798–6805. DOI: 10.1167/iovs.13-12362. PMID: 24045989.
Article49. Lee JH, O'Keefe JH, Bell D, Hensrud DD, Holick MF. 2008; Vitamin D deficiency an important, common, and easily treatable cardiovascular risk factor? J Am Coll Cardiol. 52:1949–1956. DOI: 10.1016/j.jacc.2008.08.050. PMID: 19055985.50. Yang L, Froio RM, Sciuto TE, Dvorak AM, Alon R, Luscinskas FW. 2005; ICAM-1 regulates neutrophil adhesion and transcellular migration of TNF-alpha-activated vascular endothelium under flow. Blood. 106:584–592. DOI: 10.1182/blood-2004-12-4942. PMID: 15811956. PMCID: PMC1635241.51. Zhou P, Lu S, Luo Y, Wang S, Yang K, Zhai Y, Sun G, Sun X. 2017; Attenuation of TNF-α-induced inflammatory injury in endothelial cells by ginsenoside Rb1 via inhibiting NF-κB, JNK and p38 signaling pathways. Front Pharmacol. 8:464. DOI: 10.3389/fphar.2017.00464. PMID: 28824425. PMCID: PMC5540891.
Article52. Pober JS. 2002; Endothelial activation: intracellular signaling pathways. Arthritis Res. 4(Suppl 3):S109–S116. DOI: 10.1186/ar576. PMID: 12110129. PMCID: PMC3240152.53. Springer TA. 1994; Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 76:301–314. DOI: 10.1016/0092-8674(94)90337-9. PMID: 7507411.
Article54. Blann A. 1993; von Willebrand factor and the endothelium in vascular disease. Br J Biomed Sci. 50:125–134. PMID: 8219918.55. Li X, Li J, Li Z, Sang Y, Niu Y, Zhang Q, Ding H, Yin S. 2016; Fucoidan from Undaria pinnatifida prevents vascular dysfunction through PI3K/Akt/eNOS-dependent mechanisms in the l-NAME-induced hypertensive rat model. Food Funct. 7:2398–2408. DOI: 10.1039/C6FO00288A. PMID: 27153123.
Article56. Atochin DN, Wang A, Liu VW, Critchlow JD, Dantas AP, Looft-Wilson R, Murata T, Salomone S, Shin HK, Ayata C, Moskowitz MA, Michel T, Sessa WC, Huang PL. 2007; The phosphorylation state of eNOS modulates vascular reactivity and outcome of cerebral ischemia in vivo. J Clin Invest. 117:1961–1967. DOI: 10.1172/JCI29877. PMID: 17557122. PMCID: PMC1884686.
Article57. Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. 1999; Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 399:597–601. DOI: 10.1038/21218. PMID: 10376602. PMCID: PMC3637917.
Article58. Wang Q, Zhang M, Ding Y, Wang Q, Zhang W, Song P, Zou MH. 2014; Activation of NAD(P)H oxidase by tryptophan-derived 3-hydroxykynurenine accelerates endothelial apoptosis and dysfunction in vivo. Circ Res. 114:480–492. DOI: 10.1161/CIRCRESAHA.114.302113. PMID: 24281189. PMCID: PMC4104160.
Article59. Park S, Lakatta EG. 2012; Role of inflammation in the pathogenesis of arterial stiffness. Yonsei Med J. 53:258–261. DOI: 10.3349/ymj.2012.53.2.258. PMID: 22318811. PMCID: PMC3282971.
Article60. Sen S, Roy S, Bandyopadhyay G, Scott B, Xiao D, Ramadoss S, Mahata SK, Chaudhuri G. 2016; γ-Aminobutyric acid is synthesized and released by the endothelium: potential implications. Circ Res. 119:621–634. DOI: 10.1161/CIRCRESAHA.116.308645. PMID: 27354210.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Barriers in Salt Reduction Strategies: Time to Acting for the Future
- Altered vascular calcium regulation in hypertension
- Effects of poly-gamma-glutamic acid on serum and brain concentrations of glutamate and GABA in diet-induced obese rats
- Compliance with Low-Salt Diet and Related Factors in Essential Hypertension Patients
- Anti-Hypertensive Effect of a Solar Salt Diet in Elderly Hypertensive Patients: A Preliminary Randomized, Double-Blind Clinical Trial