J Korean Med Sci.
2020 Apr;35(13):e81. 10.3346/jkms.2020.35.e81.
Objective Verification of Acute Tinnitus and Validation of Efficacy of Systemic Steroids in Rats
- Affiliations
-
- 1Department of Otolaryngology, Ajou University School of Medicine, Suwon, Korea.
- 2Department of Biomedical Sciences, BK21 Plus Research Center for Biomedical Sciences, Ajou University Graduate School of Medicine, Suwon, Korea.
- 3Department of Otorhinolaryngology Head and Neck Surgery, Dongtan Sacred Heart Hospital, Hallym University College of Medicine, Hwaseong, Korea.
- 4Department of Biomedical Engineering, College of Medicine, Dankook University, Cheonan, Korea.
- KMID: 2509604
- DOI: http://doi.org/10.3346/jkms.2020.35.e81
Abstract
- Background
This study was performed to identify acute tinnitus and evaluate the efficacy of steroids for noise-induced acute tinnitus by measuring the gap-prepulse inhibition of the acoustic startle (GPIAS) value in an animal model.
Methods
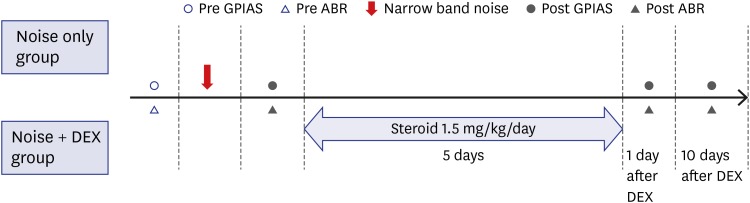
Nineteen rats (the noise group [n = 7] and the noise + dexamethasone [DEX] group [n = 12]) were exposed to narrow-band noise centered at 16 kHz from a sound generator for 4 hours. The noise + DEX group received intraperitoneal steroid administration daily for 5 days (1.5 mg/kg/day) after completing noise exposure. Auditory brainstem response and GPIAS value were measured just prior to, and 1 day after noise exposure and on days 1 and 10 days after completing steroid administration. The changes in cochlear structure were evaluated by histological analysis.
Results
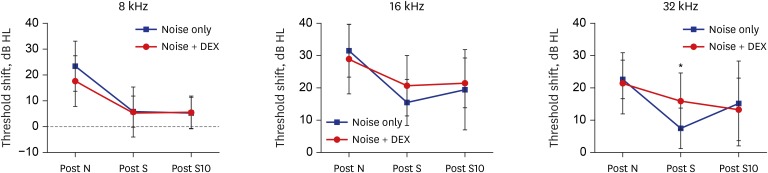
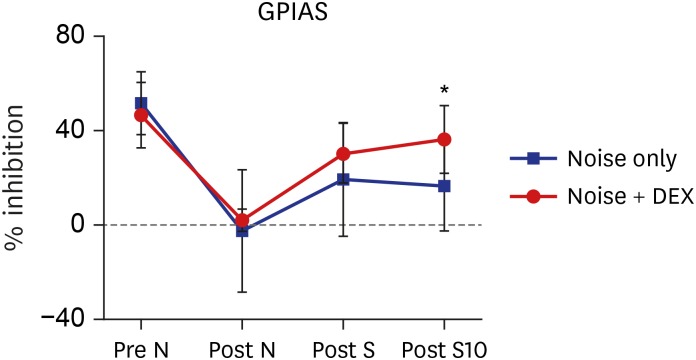
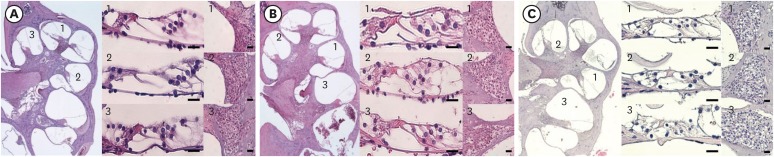
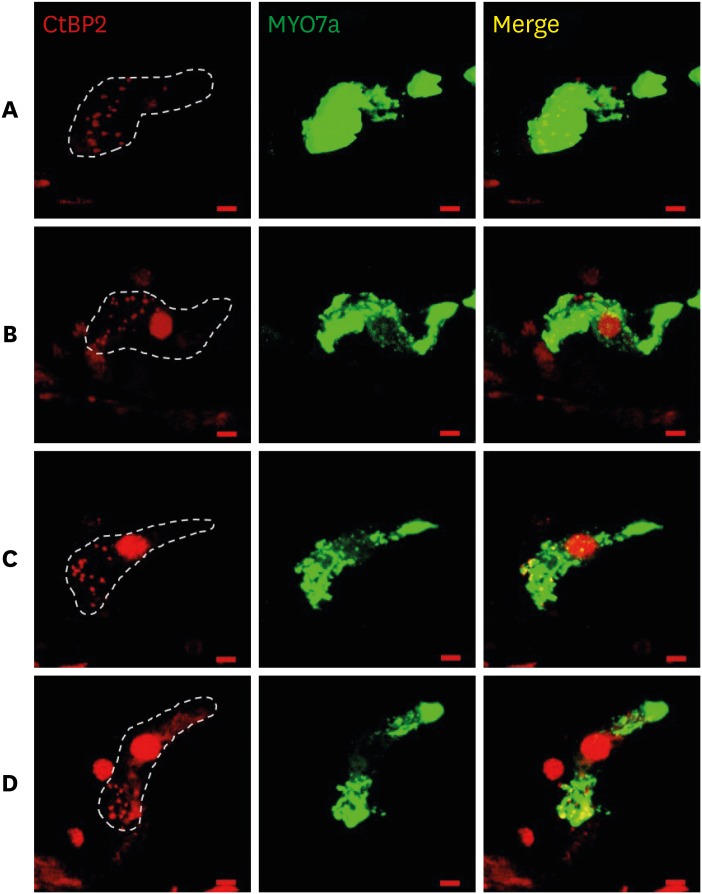
The threshold shift was checked 1 and 10 days after intraperitoneal steroid injection, and no differences in threshold shift were observed between the two groups in each frequency except for 32 kHz 1 day after steroid injection. The mean GPIAS value in the noise + DEX group (36.4% ± 14.1%) was significantly higher than that in the noise group (16.4% ± 18.8%) 10 days after intraperitoneal steroid administration (P = 0.017). There were no pathological changes associated with noise trauma in the two groups as determined on hematoxylin and eosin and immunohistochemical staining.
Conclusion
An acute tinnitus model with minimal structural changes by noise exposure was set up, and used to verify tinnitus objectively by measuring the GPIAS value. Steroid therapy for control of tinnitus was validated in this animal model.
Figure
Reference
-
1. Henry JA, Dennis KC, Schechter MA. General review of tinnitus: prevalence, mechanisms, effects, and management. J Speech Lang Hear Res. 2005; 48(5):1204–1235. PMID: 16411806.2. Henry JA, Zaugg TL, Myers PJ, Schechter MA. The role of audiologic evaluation in progressive audiologic tinnitus management. Trends Amplif. 2008; 12(3):170–187. PMID: 18628281.
Article3. Kimura M, Eggermont JJ. Effects of acute pure tone induced hearing loss on response properties in three auditory cortical fields in cat. Hear Res. 1999; 135(1-2):146–162. PMID: 10491963.
Article4. Manabe Y, Yoshida S, Saito H, Oka H. Effects of lidocaine on salicylate-induced discharge of neurons in the inferior colliculus of the guinea pig. Hear Res. 1997; 103(1-2):192–198. PMID: 9007584.
Article5. Bauer CA, Turner JG, Caspary DM, Myers KS, Brozoski TJ. Tinnitus and inferior colliculus activity in chinchillas related to three distinct patterns of cochlear trauma. J Neurosci Res. 2008; 86(11):2564–2578. PMID: 18438941.
Article6. Kaltenbach JA, Godfrey DA, Neumann JB, McCaslin DL, Afman CE, Zhang J. Changes in spontaneous neural activity in the dorsal cochlear nucleus following exposure to intense sound: relation to threshold shift. Hear Res. 1998; 124(1-2):78–84. PMID: 9822904.
Article7. Jastreboff PJ, Brennan JF, Coleman JK, Sasaki CT. Phantom auditory sensation in rats: an animal model for tinnitus. Behav Neurosci. 1988; 102(6):811–822. PMID: 3214530.
Article8. Kaltenbach JA, Zacharek MA, Zhang J, Frederick S. Activity in the dorsal cochlear nucleus of hamsters previously tested for tinnitus following intense tone exposure. Neurosci Lett. 2004; 355(1-2):121–125. PMID: 14729250.
Article9. Heffner HE, Harrington IA. Tinnitus in hamsters following exposure to intense sound. Hear Res. 2002; 170(1-2):83–95. PMID: 12208543.
Article10. Galazyuk A, Hébert S. Gap-prepulse inhibition of the acoustic startle reflex (GPIAS) for tinnitus assessment: current status and future directions. Front Neurol. 2015; 6:88. PMID: 25972836.
Article11. Kaltenbach JA. Tinnitus: models and mechanisms. Hear Res. 2011; 276(1-2):52–60. PMID: 21146597.
Article12. Turner JG, Brozoski TJ, Bauer CA, Parrish JL, Myers K, Hughes LF, et al. Gap detection deficits in rats with tinnitus: a potential novel screening tool. Behav Neurosci. 2006; 120(1):188–195. PMID: 16492129.
Article13. Park YM, Na WS, Park IY, Suh MW, Rhee CK, Chung PS, et al. Trans-canal laser irradiation reduces tinnitus perception of salicylate treated rat. Neurosci Lett. 2013; 544:131–135. PMID: 23583341.
Article14. Ochi K, Ohashi T, Kenmochi M. Hearing impairment and tinnitus pitch in patients with unilateral tinnitus: comparison of sudden hearing loss and chronic tinnitus. Laryngoscope. 2003; 113(3):427–431. PMID: 12616191.
Article15. Hu A, Parnes LS. Intratympanic steroids for inner ear disorders: a review. Audiol Neurootol. 2009; 14(6):373–382. PMID: 19923807.
Article16. Liberman MC, Dodds LW, Pierce S. Afferent and efferent innervation of the cat cochlea: quantitative analysis with light and electron microscopy. J Comp Neurol. 1990; 301(3):443–460. PMID: 2262601.
Article17. Parnes SM. Current concepts in the clinical management of patients with tinnitus. Eur Arch Otorhinolaryngol. 1997; 254(9-10):406–409. PMID: 9438106.
Article18. Langguth B, Elgoyhen AB. Current pharmacological treatments for tinnitus. Expert Opin Pharmacother. 2012; 13(17):2495–2509. PMID: 23121658.
Article19. ten Cate WJ, Curtis LM, Small GM, Rarey KE. Localization of glucocorticoid receptors and glucocorticoid receptor mRNAs in the rat cochlea. Laryngoscope. 1993; 103(8):865–871. PMID: 8361289.
Article20. Shim HJ, Song SJ, Choi AY, Hyung Lee R, Yoon SW. Comparison of various treatment modalities for acute tinnitus. Laryngoscope. 2011; 121(12):2619–2625. PMID: 22109762.
Article21. van de Heyning P, Muehlmeier G, Cox T, Lisowska G, Maier H, Morawski K, et al. Efficacy and safety of AM-101 in the treatment of acute inner ear tinnitus--a double-blind, randomized, placebo-controlled phase II study. Otol Neurotol. 2014; 35(4):589–597. PMID: 24603353.
Article22. Lee HJ, Kim MB, Yoo SY, Park SN, Nam EC, Moon IS, et al. Clinical effect of intratympanic dexamethasone injection in acute unilateral tinnitus: a prospective, placebo-controlled, multicenter study. Laryngoscope. 2018; 128(1):184–188. PMID: 28224644.
Article23. Yamashita D, Jiang HY, Schacht J, Miller JM. Delayed production of free radicals following noise exposure. Brain Res. 2004; 1019(1-2):201–209. PMID: 15306254.
Article24. Greijer AE, van der Wall E. The role of hypoxia inducible factor 1 (HIF-1) in hypoxia induced apoptosis. J Clin Pathol. 2004; 57(10):1009–1014. PMID: 15452150.
Article25. Yilmaz I, Yilmazer C, Erkan AN, Aslan SG, Ozluoglu LN. Intratympanic dexamethasone injection effects on transient-evoked otoacoustic emission. Am J Otolaryngol. 2005; 26(2):113–117. PMID: 15742264.
Article26. Sanchez TG, Medeiros IR, Levy CP, Ramalho Jda R, Bento RF. Tinnitus in normally hearing patients: clinical aspects and repercussions. Rev Bras Otorrinolaringol (Engl Ed). 2005; 71(4):427–431.
Article27. Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci. 2009; 29(45):14077–14085. PMID: 19906956.
Article28. Sakata E, Itoh A, Itoh Y. Treatment of cochlear-tinnitus with dexamethasone infusion into the tympanic cavity. Int Tinnitus J. 1996; 2:129–135. PMID: 10753351.