J Korean Med Sci.
2020 Dec;35(48):e405. 10.3346/jkms.2020.35.e405.
Tandem High-dose Chemotherapy without Craniospinal Irradiation in Treatment of Non-metastatic Malignant Brain Tumors in Very Young Children
- Affiliations
-
- 1Department of Pediatrics, Seoul National University Bundang Hospital, Seongnam, Korea
- 2Department of Radiation Oncology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 3Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 4Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 5Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2509526
- DOI: http://doi.org/10.3346/jkms.2020.35.e405
Abstract
- Background
Infants and very young children with malignant brain tumors have a poorer survival and a higher risk for neurologic deficits. The present study evaluated the feasibility and effectiveness of multimodal treatment including tandem high-dose chemotherapy and autologous stem cell transplantation (HDCT/auto-SCT) in minimizing use of radiotherapy (RT) in very young children with non-metastatic malignant brain tumors.
Methods
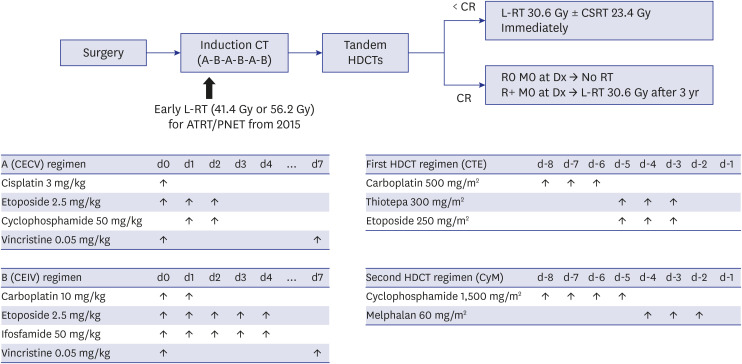
Twenty consecutive patients younger than 3 years were enrolled between 2004 and 2017. Tandem HDCT/auto-SCT was performed after six cycles of induction chemotherapy. Local RT was administered only to patients with post-operative gross residual tumor at older than 3 years. Since September 2015, early post-operative local RT for patients with atypical teratoid/rhabdoid tumor or primitive neuroectodermal tumor was administered.
Results
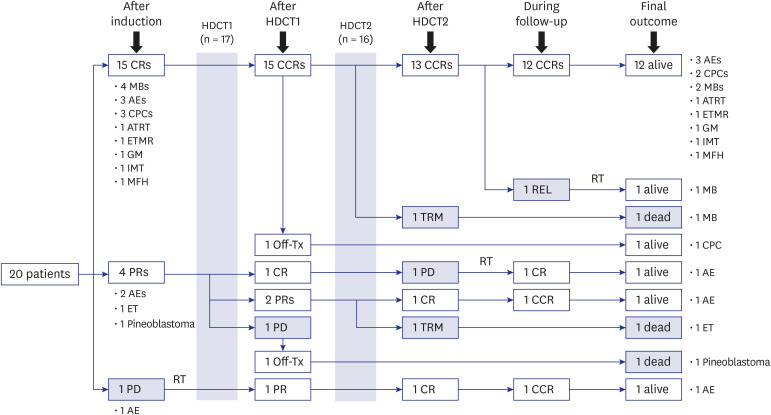
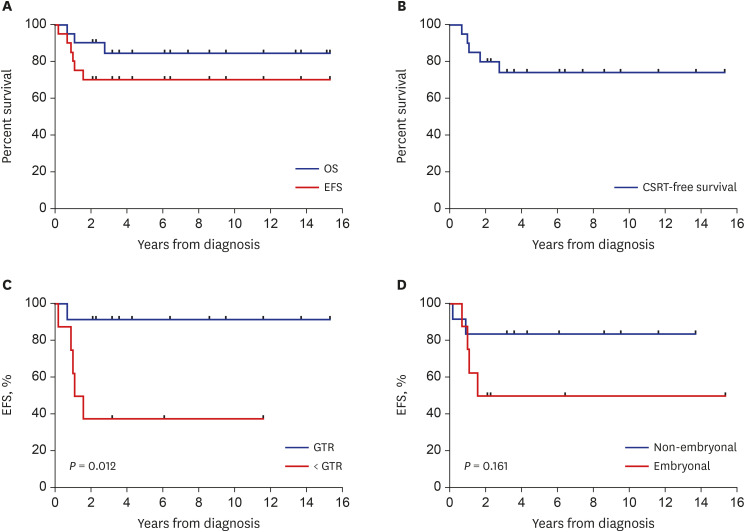
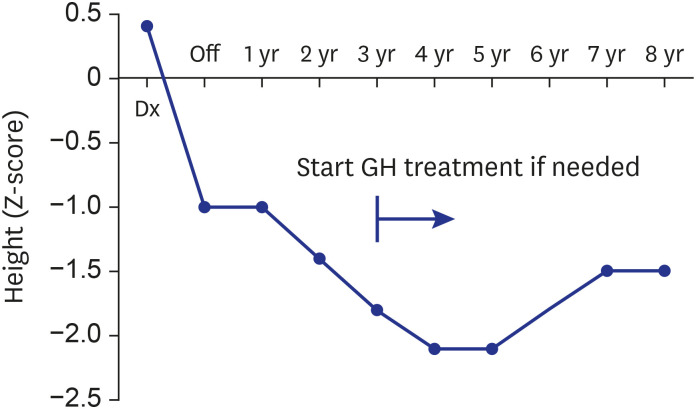
All 20 enrolled patients underwent the first HDCT/auto-SCT, and 18 proceeded to the second. Two patients died from toxicity during the second HDCT/auto-SCT, and four patients experienced relapse/progression (one localized and three metastatic), three of whom remained alive after salvage treatment including RT. A total of 17 patients remained alive at a median 7.8 (range, 2.5−15.7) years from diagnosis. Nine survivors received no RT, six survivors received local RT alone, and two survivors who experienced metastatic relapse after tandem HDCT/auto-SCT received both local and craniospinal RT. The 5-year overall, eventfree, and craniospinal RT-free survival rates were 85.0% ± 8.0%, 70.0% ± 10.2%, and 75.0% ± 9.7%, respectively. Neuroendocrine and neurocognitive functions evaluated 5 years after tandem HDCT/auto-SCT were acceptable.
Conclusion
Our results suggest that non-metastatic malignant brain tumors in very young children could be treated with multimodal therapy including tandem HDCT/auto-SCT while minimizing RT, particularly craniospinal RT.
Figure
Cited by 1 articles
-
Tandem High-Dose Chemotherapy Increases the Risk of Secondary Malignant Neoplasm in Pediatric Solid Tumors
Hana Lim, Minji Im, Eun Seop Seo, Hee Won Cho, Hee Young Ju, Keon Hee Yoo, Sung Yoon Cho, Jong-Won Kim, Do Hoon Lim, Ki Woong Sung, Ji Won Lee
Cancer Res Treat. 2024;56(2):642-651. doi: 10.4143/crt.2023.999.
Reference
-
1. Sung KW, Lim DH, Lee SH, Yoo KH, Koo HH, Kim JH, et al. Tandem high-dose chemotherapy and auto-SCT for malignant brain tumors in children under 3 years of age. Bone Marrow Transplant. 2013; 48(7):932–938. PMID: 23318534.
Article2. Duffner PK, Horowitz ME, Krischer JP, Friedman HS, Burger PC, Cohen ME, et al. Postoperative chemotherapy and delayed radiation in children less than three years of age with malignant brain tumors. N Engl J Med. 1993; 328(24):1725–1731. PMID: 8388548.
Article3. Geyer JR, Zeltzer PM, Boyett JM, Rorke LB, Stanley P, Albright AL, et al. Survival of infants with primitive neuroectodermal tumors or malignant ependymomas of the CNS treated with eight drugs in 1 day: a report from the Childrens Cancer Group. J Clin Oncol. 1994; 12(8):1607–1615. PMID: 8040673.
Article4. Grill J, Sainte-Rose C, Jouvet A, Gentet JC, Lejars O, Frappaz D, et al. Treatment of medulloblastoma with postoperative chemotherapy alone: an SFOP prospective trial in young children. Lancet Oncol. 2005; 6(8):573–580. PMID: 16054568.
Article5. Berthold F, Boos J, Burdach S, Erttmann R, Henze G, Hermann J, et al. Myeloablative megatherapy with autologous stem-cell rescue versus oral maintenance chemotherapy as consolidation treatment in patients with high-risk neuroblastoma: a randomised controlled trial. Lancet Oncol. 2005; 6(9):649–658. PMID: 16129365.
Article6. Marachelian A, Butturini A, Finlay J. Myeloablative chemotherapy with autologous hematopoietic progenitor cell rescue for childhood central nervous system tumors. Bone Marrow Transplant. 2008; 41(2):167–172. PMID: 18176620.
Article7. Matthay KK, Reynolds CP, Seeger RC, Shimada H, Adkins ES, Haas-Kogan D, et al. Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: a children’s oncology group study. J Clin Oncol. 2009; 27(7):1007–1013. PMID: 19171716.
Article8. Mason WP, Grovas A, Halpern S, Dunkel IJ, Garvin J, Heller G, et al. Intensive chemotherapy and bone marrow rescue for young children with newly diagnosed malignant brain tumors. J Clin Oncol. 1998; 16(1):210–221. PMID: 9440745.
Article9. Chi SN, Gardner SL, Levy AS, Knopp EA, Miller DC, Wisoff JH, et al. Feasibility and response to induction chemotherapy intensified with high-dose methotrexate for young children with newly diagnosed high-risk disseminated medulloblastoma. J Clin Oncol. 2004; 22(24):4881–4887. PMID: 15611503.
Article10. Dhall G, Grodman H, Ji L, Sands S, Gardner S, Dunkel IJ, et al. Outcome of children less than three years old at diagnosis with non-metastatic medulloblastoma treated with chemotherapy on the “Head Start” I and II protocols. Pediatr Blood Cancer. 2008; 50(6):1169–1175. PMID: 18293379.
Article11. Zaky W, Dhall G, Khatua S, Brown RJ, Ginn KF, Gardner SL, et al. Choroid plexus carcinoma in children: the Head Start experience. Pediatr Blood Cancer. 2015; 62(5):784–789. PMID: 25662896.
Article12. Espinoza JC, Haley K, Patel N, Dhall G, Gardner S, Allen J, et al. Outcome of young children with high-grade glioma treated with irradiation-avoiding intensive chemotherapy regimens: final report of the Head Start II and III trials. Pediatr Blood Cancer. 2016; 63(10):1806–1813. PMID: 27332770.
Article13. Cohen BH, Geyer JR, Miller DC, Curran JG, Zhou T, Holmes E, et al. Pilot study of intensive chemotherapy with peripheral hematopoietic cell support for children less than 3 years of age with malignant brain tumors, the CCG-99703 phase I/II study. A report from the Children's Oncology Group. Pediatr Neurol. 2015; 53(1):31–46. PMID: 26092413.14. Lafay-Cousin L, Smith A, Chi SN, Wells E, Madden J, Margol A, et al. Clinical, pathological, and molecular characterization of infant medulloblastomas treated with sequential high-dose chemotherapy. Pediatr Blood Cancer. 2016; 63(9):1527–1534. PMID: 27145464.
Article15. Northcott PA, Shih DJ, Remke M, Cho YJ, Kool M, Hawkins C, et al. Rapid, reliable, and reproducible molecular sub-grouping of clinical medulloblastoma samples. Acta Neuropathol. 2012; 123(4):615–626. PMID: 22057785.
Article16. Raleigh DR, Tomlin B, Buono BD, Roddy E, Sear K, Byer L, et al. Survival after chemotherapy and stem cell transplant followed by delayed craniospinal irradiation is comparable to upfront craniospinal irradiation in pediatric embryonal brain tumor patients. J Neurooncol. 2017; 131(2):359–368. PMID: 27778212.
Article17. Ashley DM, Merchant TE, Strother D, Zhou T, Duffner P, Burger PC, et al. Induction chemotherapy and conformal radiation therapy for very young children with nonmetastatic medulloblastoma: Children's Oncology Group study P9934. J Clin Oncol. 2012; 30(26):3181–3186. PMID: 22851568.
Article18. Pompe RS, von Bueren AO, Mynarek M, von Hoff K, Friedrich C, Kwiecien R, et al. Intraventricular methotrexate as part of primary therapy for children with infant and/or metastatic medulloblastoma: feasibility, acute toxicity and evidence for efficacy. Eur J Cancer. 2015; 51(17):2634–2642. PMID: 26346136.
Article19. Blaney SM, Tagen M, Onar-Thomas A, Berg SL, Gururangan S, Scorsone K, et al. A phase-1 pharmacokinetic optimal dosing study of intraventricular topotecan for children with neoplastic meningitis: a Pediatric Brain Tumor Consortium study. Pediatr Blood Cancer. 2013; 60(4):627–632. PMID: 23002039.
Article20. Yamada A, Moritake H, Kamimura S, Yamashita S, Takeshima H, Nunoi H. Proposed strategy for the use of high-dose chemotherapy with stem cell rescue and intrathecal topotecan without whole-brain irradiation for infantile classic medulloblastoma. Pediatr Blood Cancer. 2014; 61(12):2316–2318. PMID: 25174961.
Article21. Lee SH, Son MH, Sung KW, Choi YB, Lee NH, Yoo KH, et al. Toxicity of tandem high-dose chemotherapy and autologous stem cell transplantation using carboplatin-thiotepa-etoposide and cyclophosphamide-melphalan regimens for malignant brain tumors in children and young adults. J Neurooncol. 2014; 120(3):507–513. PMID: 25108776.
Article22. von Bueren AO, von Hoff K, Pietsch T, Gerber NU, Warmuth-Metz M, Deinlein F, et al. Treatment of young children with localized medulloblastoma by chemotherapy alone: results of the prospective, multicenter trial HIT 2000 confirming the prognostic impact of histology. Neuro-oncol. 2011; 13(6):669–679. PMID: 21636711.
Article23. AbdelBaki MS, Boué DR, Finlay JL, Kieran MW. Desmoplastic nodular medulloblastoma in young children: a management dilemma. Neuro-oncol. 2018; 20(8):1026–1033. PMID: 29156007.
Article24. Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization Classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016; 131(6):803–820. PMID: 27157931.
Article25. Abu Arja MH, Bouffet E, Finlay JL, AbdelBaki MS. Critical review of the management of primary central nervous nongerminomatous germ cell tumors. Pediatr Blood Cancer. 2019; 66(6):e27658. PMID: 30767415.
Article26. Ogawa K, Toita T, Nakamura K, Uno T, Onishi H, Itami J, et al. Treatment and prognosis of patients with intracranial nongerminomatous malignant germ cell tumors: a multiinstitutional retrospective analysis of 41 patients. Cancer. 2003; 98(2):369–376. PMID: 12872359.27. Lee YH, Park EK, Park YS, Shim KW, Choi JU, Kim DS. Treatment and outcomes of primary intracranial teratoma. Childs Nerv Syst. 2009; 25(12):1581–1587. PMID: 19693515.
Article28. Phi JH, Kim SK, Park SH, Hong SH, Wang KC, Cho BK. Immature teratomas of the central nervous system: is adjuvant therapy mandatory? J Neurosurg. 2005; 103(6):Suppl. 524–530. PMID: 16383251.
Article29. Huang X, Zhang R, Zhou LF. Diagnosis and treatment of intracranial immature teratoma. Pediatr Neurosurg. 2009; 45(5):354–360. PMID: 19907199.
Article30. Grill J, Le Deley MC, Gambarelli D, Raquin MA, Couanet D, Pierre-Kahn A, et al. Postoperative chemotherapy without irradiation for ependymoma in children under 5 years of age: a multicenter trial of the French Society of Pediatric Oncology. J Clin Oncol. 2001; 19(5):1288–1296. PMID: 11230470.31. Grundy RG, Wilne SA, Weston CL, Robinson K, Lashford LS, Ironside J, et al. Primary postoperative chemotherapy without radiotherapy for intracranial ependymoma in children: the UKCCSG/SIOP prospective study. Lancet Oncol. 2007; 8(8):696–705. PMID: 17644039.
Article32. Needle MN, Goldwein JW, Grass J, Cnaan A, Bergman I, Molloy P, et al. Adjuvant chemotherapy for the treatment of intracranial ependymoma of childhood. Cancer. 1997; 80(2):341–347. PMID: 9217048.
Article33. Geyer JR, Sposto R, Jennings M, Boyett JM, Axtell RA, Breiger D, et al. Multiagent chemotherapy and deferred radiotherapy in infants with malignant brain tumors: a report from the Children's Cancer Group. J Clin Oncol. 2005; 23(30):7621–7631. PMID: 16234523.
Article34. Foreman NK, Love S, Gill SS, Coakham HB. Second-look surgery for incompletely resected fourth ventricle ependymomas: technical case report. Neurosurgery. 1997; 40(4):856–860. PMID: 9092863.
Article35. Lee JW, Lim DH, Sung KW, Lee HJ, Yi ES, Yoo KH, et al. Multimodal treatment including tandem high-dose chemotherapy and autologous stem cell transplantation in children with anaplastic ependymomas. Pediatr Transplant. 2018; 22(3):e13127. PMID: 29453811.
Article36. Sung KW, Lim DH, Lee SH, Yoo KH, Koo HH, Kim JH, et al. Tandem high-dose chemotherapy and autologous stem cell transplantation for anaplastic ependymoma in children younger than 3 years of age. J Neurooncol. 2012; 107(2):335–342. PMID: 22081297.37. Rajagopal R, Foo JC, Jawin V, Qaddoumi I, Bouffet E. High-dose chemotherapy with autologous stem cell transplantation in infants and young children with ependymoma: a 10-year experience with the Head Start II protocol. Pediatr Transplant. 2019; 23(4):e13421. PMID: 31012212.
Article38. Merchant TE, Bendel AE, Sabin ND, Burger PC, Shaw DW, Chang E, et al. Conformal radiation therapy for pediatric ependymoma, chemotherapy for incompletely resected ependymoma, and observation for completely resected, supratentorial ependymoma. J Clin Oncol. 2019; 37(12):974–983. PMID: 30811284.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- High-dose chemotherapy with reduced-dose craniospinal radiotherapy in children with newly diagnosed high-risk brain tumor
- Malignant Brain Tumours in Children : Present and Future Perspectives
- Tandem High-dose Chemotherapy and Autologous Stem Cell Transplantation in Children with Brain Tumors : Review of Single Center Experience
- Tandem High-Dose Chemotherapy and Autologous Stem Cell Transplantation for Atypical Teratoid/Rhabdoid Tumor
- Understanding the Treatment Strategies of Intracranial Germ Cell Tumors: Focusing on Radiotherapy