Korean J Sports Med.
2020 Dec;38(4):217-224. 10.5763/kjsm.2020.38.4.217.
Biological Enhancement of Healing with Kartogenin Injection at the Tendon-to-Bone Interface in a Rat Rotator Cuff Tear Model
- Affiliations
-
- 1Department of Orthopaedic Surgery, Kyungpook National University Hospital, Daegu, Korea
- 2Department of Bio-Fibers and Materials Science, College of Agriculture and Life Science, Kyungpook National University, Daegu, Korea
- KMID: 2509020
- DOI: http://doi.org/10.5763/kjsm.2020.38.4.217
Abstract
- Purpose
The purpose of this study was to investigate the effects of kartogenin (KGN) on the tendon-bone interface in a rat rotator cuff tear model.
Methods
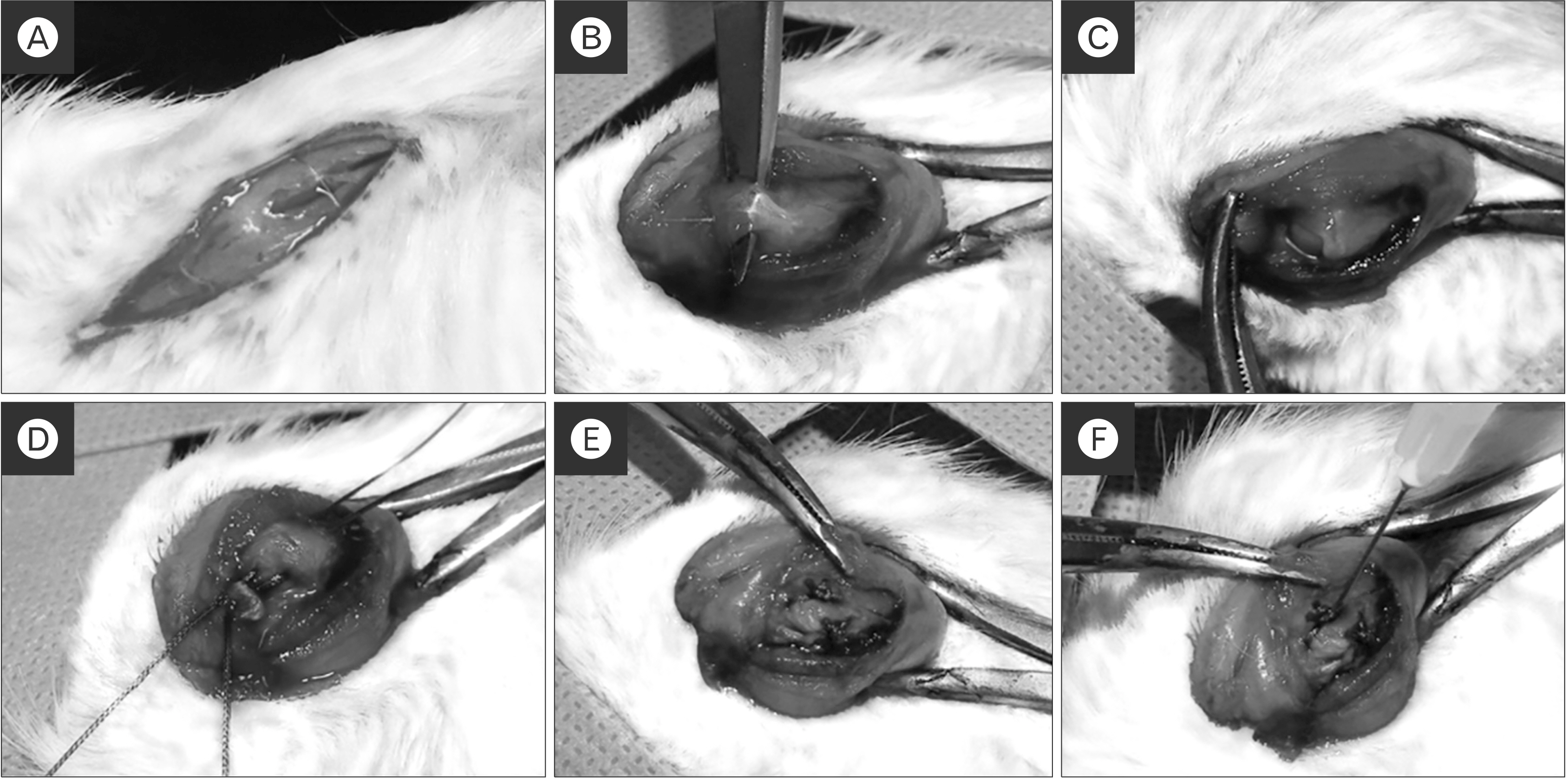
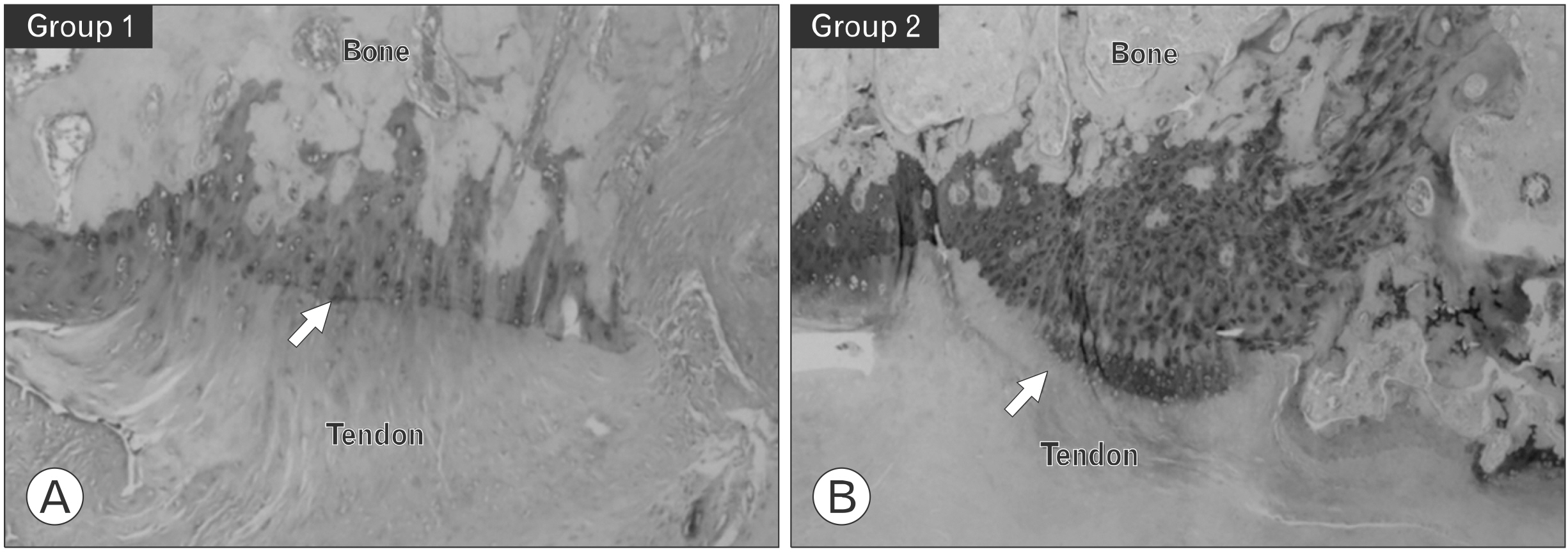
Twenty male rats were divided into two equal groups; group 1 (repair only) and group 2 (KGN single injection). A rat rotator cuff rupture model was prepared, and KGN (500 μM) was injected into the repair site. The specimens were collected after 8 weeks, and biomechanical and histological analyses were performed. We assessed the healing quality of the tendon-to-bone repair site using six aspects of tendon tissue. The histological findings were classified semiquantitatively into four grades (grade 0, the poorest appearance; grade 1, poorer; grade 2, better; and grade 3, marked regeneration).
Results
Group 2 showed a higher mean ultimate load to failure than the control group (group 1: 25.78±31.38 N, group 2: 55.64±36.02 N; p=0.011). On histological analysis, group 2 exhibited a significantly greater total score (group 1: 7.20±2.14, group 2: 9.50±1.84; p=0.019), collagen fiber continuity (group 1: 1.20±0.42, group 2: 1.70±0.48; p=0.024), and collagen fiber density (group 1: 1.50±0.52, group 2: 2.20±0.63; p=0.080). Metachromasia were more intense in group 2 than in the control group.
Conclusion
A single injection of KGN reinforces biomechanical strength and the formation of collagen and fibrocartilage at the tendon-to-bone interface in a rat rotator cuff tear model.
Keyword
Figure
Reference
-
1. Yamaguchi K, Ditsios K, Middleton WD, Hildebolt CF, Galatz LM, Teefey SA. 2006; The demographic and morphological features of rotator cuff disease: a comparison of asymptomatic and symptomatic shoulders. J Bone Joint Surg Am. 88:1699–704. DOI: 10.2106/00004623-200608000-00002. PMID: 16882890.2. Wang VM, Wang FC, McNickle AG, et al. 2010; Medial versus lateral supraspinatus tendon properties: implications for double- row rotator cuff repair. Am J Sports Med. 38:2456–63. DOI: 10.1177/0363546510376817. PMID: 20929937. PMCID: PMC3772634.3. Le BT, Wu XL, Lam PH, Murrell GA. 2014; Factors predicting rotator cuff retears: an analysis of 1000 consecutive rotator cuff repairs. Am J Sports Med. 42:1134–42. DOI: 10.1177/0363546514525336. PMID: 24748610.4. Castricini R, Longo UG, De Benedetto M, et al. 2011; Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: a randomized controlled trial. Am J Sports Med. 39:258–65. DOI: 10.1177/0363546510390780. PMID: 21160018.5. Wang LL, Yin XF, Chu XC, Zhang YB, Gong XN. 2018; Platelet-derived growth factor subunit B is required for tendon-bone healing using bone marrow-derived mesenchymal stem cells after rotator cuff repair in rats. J Cell Biochem. 119:8897–908. DOI: 10.1002/jcb.27143. PMID: 30105826.
Article6. Yonemitsu R, Tokunaga T, Shukunami C, et al. 2019; Fibroblast growth factor 2 enhances tendon-to-bone healing in a rat rotator cuff repair of chronic tears. Am J Sports Med. 47:1701–12. DOI: 10.1177/0363546519836959. PMID: 31038985.
Article7. Liu Q, Yu Y, Reisdorf RL, et al. 2019; Engineered tendon- fibrocartilage-bone composite and bone marrow-derived mesenchymal stem cell sheet augmentation promotes rotator cuff healing in a non-weight-bearing canine model. Biomaterials. 192:189–98. DOI: 10.1016/j.biomaterials.2018.10.037. PMID: 30453215.8. Wang C, Hu Q, Song W, Yu W, He Y. 2020; Adipose stem cell- derived exosomes decrease fatty infiltration and enhance rotator cuff healing in a rabbit model of chronic tears. Am J Sports Med. 48:1456–64. DOI: 10.1177/0363546520908847. PMID: 32272021.9. Zhang J, Wang JH. 2014; Kartogenin induces cartilage-like tissue formation in tendon-bone junction. Bone Res. 2:14008. DOI: 10.1038/boneres.2014.8. PMID: 25419468. PMCID: PMC4237211.
Article10. Im GI. 2018; Application of kartogenin for musculoskeletal regeneration. J Biomed Mater Res A. 106:1141–8. DOI: 10.1002/jbm.a.36300. PMID: 29164815.
Article11. Mohan G, Magnitsky S, Melkus G, et al. 2016; Kartogenin treatment prevented joint degeneration in a rodent model of osteoarthritis: a pilot study. J Orthop Res. 34:1780–9. DOI: 10.1002/jor.23197. PMID: 26895619. PMCID: PMC6348064.
Article12. Xu X, Shi D, Shen Y, et al. 2015; Full-thickness cartilage defects are repaired via a microfracture technique and intraarticular injection of the small-molecule compound kartogenin. Arthritis Res Ther. 17:20. DOI: 10.1186/s13075-015-0537-1. PMID: 25641548. PMCID: PMC4376363.
Article13. Zhang J, Yuan T, Zheng N, Zhou Y, Hogan MV, Wang JH. 2017; The combined use of kartogenin and platelet-rich plasma promotes fibrocartilage formation in the wounded rat Achilles tendon entheses. Bone Joint Res. 6:231–44. DOI: 10.1302/2046-3758.64.BJR-2017-0268.R1. PMID: 28450316. PMCID: PMC5415905.
Article14. Kim DH, Min SG, Yoon JP, et al. 2019; Mechanical augmentation with absorbable alginate sheet enhances healing of the rotator cuff. Orthopedics. 42:e104–10. DOI: 10.3928/01477447-20181206-04. PMID: 30540880.
Article15. Johnson K, Zhu S, Tremblay MS, et al. 2012; A stem cell-based approach to cartilage repair. Science. 336:717–21. DOI: 10.1126/science.1215157. PMID: 22491093.
Article16. Kang ML, Ko JY, Kim JE, Im GI. 2014; Intra-articular delivery of kartogenin-conjugated chitosan nano/microparticles for cartilage regeneration. Biomaterials. 35:9984–94. DOI: 10.1016/j.biomaterials.2014.08.042. PMID: 25241157.
Article17. Wang D, Tan H, Lebaschi AH, et al. 2018; Kartogenin enhances collagen organization and mechanical strength of the repaired enthesis in a murine model of rotator cuff repair. Arthroscopy. 34:2579–87. DOI: 10.1016/j.arthro.2018.04.022. PMID: 30037570. PMCID: PMC6371391.
Article18. Huang C, Zhang X, Luo H, et al. 2020; Jul. 7. Effect of kartogenin-loaded gelatin methacryloyl hydrogel scaffold with bone marrow stimulation for enthesis healing in rotator cuff repair. J Shoulder Elbow Surg. [Epub]. https://doi.org/10.1016/. DOI: 10.1016/j.jse.2020.06.013. PMID: 32650072.
Article19. Shi D, Xu X, Ye Y, et al. 2016; Photo-cross-linked scaffold with kartogenin-encapsulated nanoparticles for cartilage regeneration. ACS Nano. 10:1292–9. DOI: 10.1021/acsnano.5b06663. PMID: 26757419.
Article20. Hu Q, Ding B, Yan X, et al. 2017; Polyethylene glycol modified PAMAM dendrimer delivery of kartogenin to induce chondrogenic differentiation of mesenchymal stem cells. Nanomedicine. 13:2189–98. DOI: 10.1016/j.nano.2017.05.011. PMID: 28579434.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current and Future Strategies to Enhance Healing at the Tendon-To-Bone Interface of a Rotator Cuff Tear
- Usefulness of Multiphase Scaffolds for Improving Tendon to Bone Healing for Rotator Cuff Tears in Shoulder
- Biological Characteristics of Rotator Cuff Tendon
- Can the Rotator Cuff Tear Be Treated with Atelocollagen?
- Selective Serotonin Reuptake Inhibitor Promotes Bone-Tendon Interface Healing in a Rotator Cuff Tear Rat Model