Int J Stem Cells.
2020 Nov;13(3):414-423. 10.15283/ijsc20049.
miR-29a in Exosomes from Bone Marrow Mesenchymal Stem Cells Inhibit Fibrosis during Endometrial Repair of Intrauterine Adhesion
- Affiliations
-
- 1Department of Gynecological Oncology, The Affiliated Changzhou Maternal and Child Health Care Hospital of Nanjing Medical University, Changzhou, China
- 2Department of Gynecology, The Second Affiliated Hospital of Nanjing Medical University, Nanjing, China
- KMID: 2508915
- DOI: http://doi.org/10.15283/ijsc20049
Abstract
- Background and Objectives
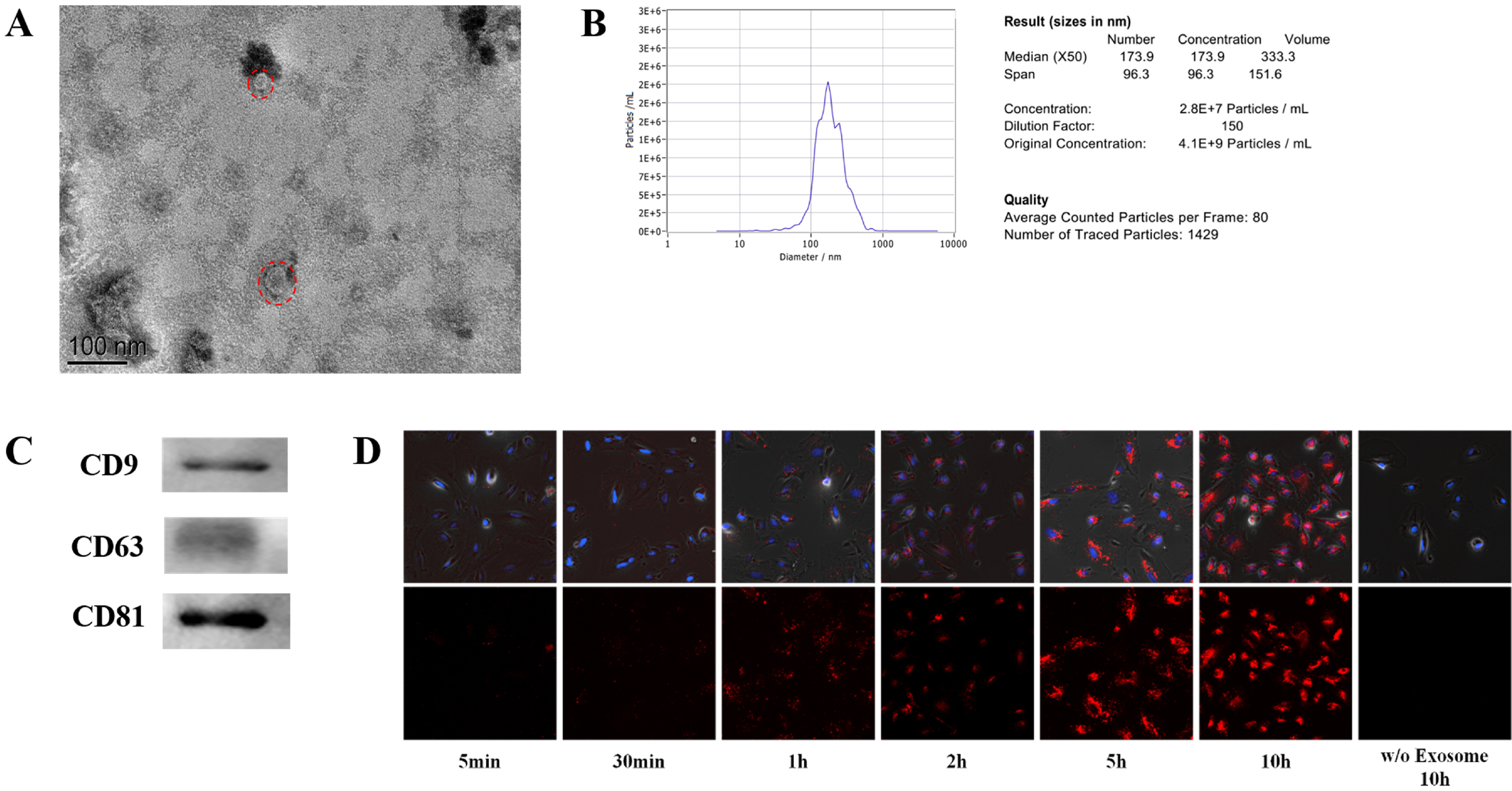
Bone marrow mesenchymal stem cells (BMSCs) is an ideal source of stem cells in the treatment of intrauterine adhesion. Exosomes are a type of membrane vesicle and the diameter is 30∼100 nm. Exosomes can take their contents into the target cells, releasing and exerting their functions. In this study, we intend to study the role of human BMSC-derived exosomes (BMSC-Exo) in promoting endometrial damage repair in the treatment of IUA.
Methods
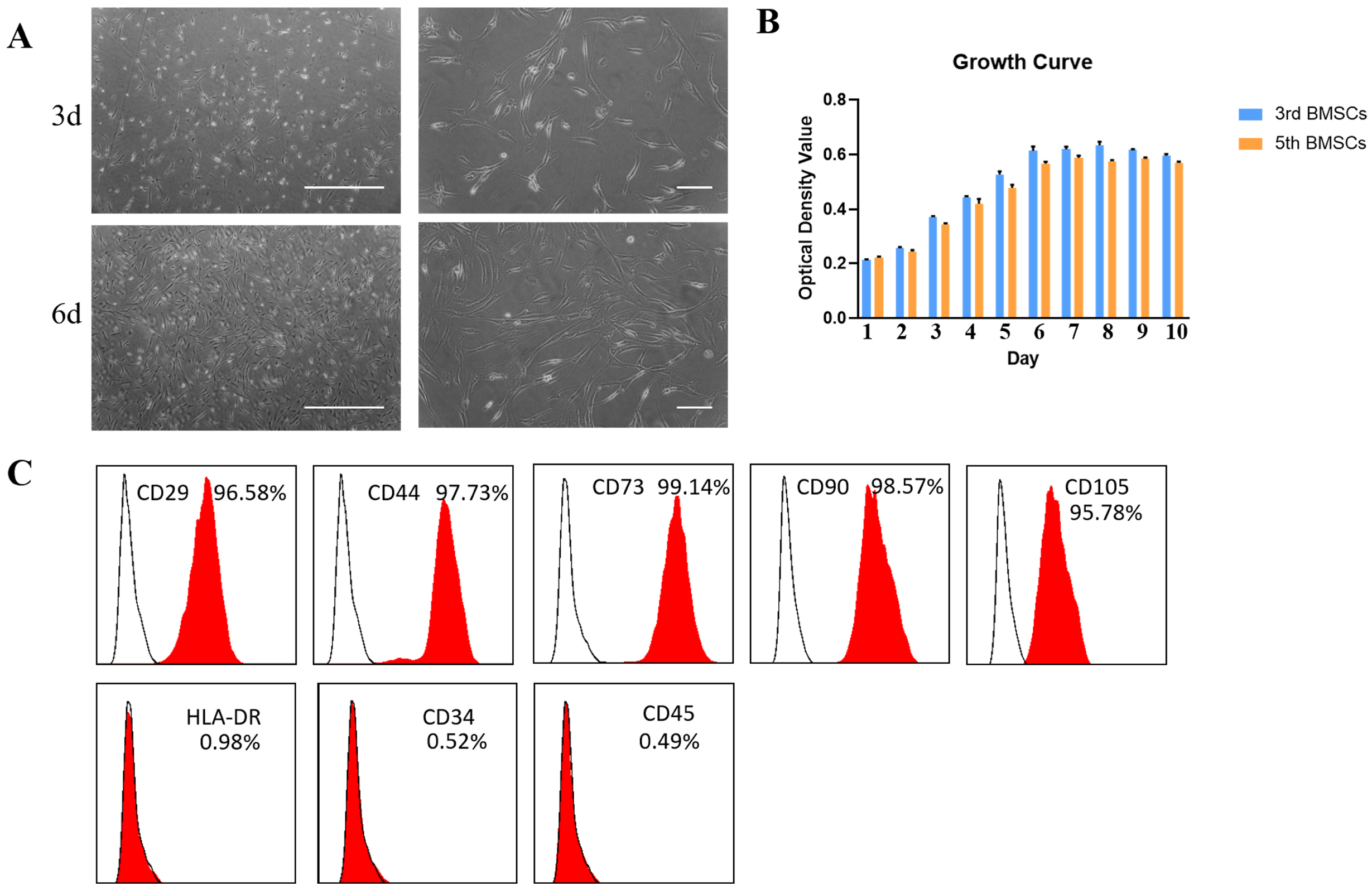
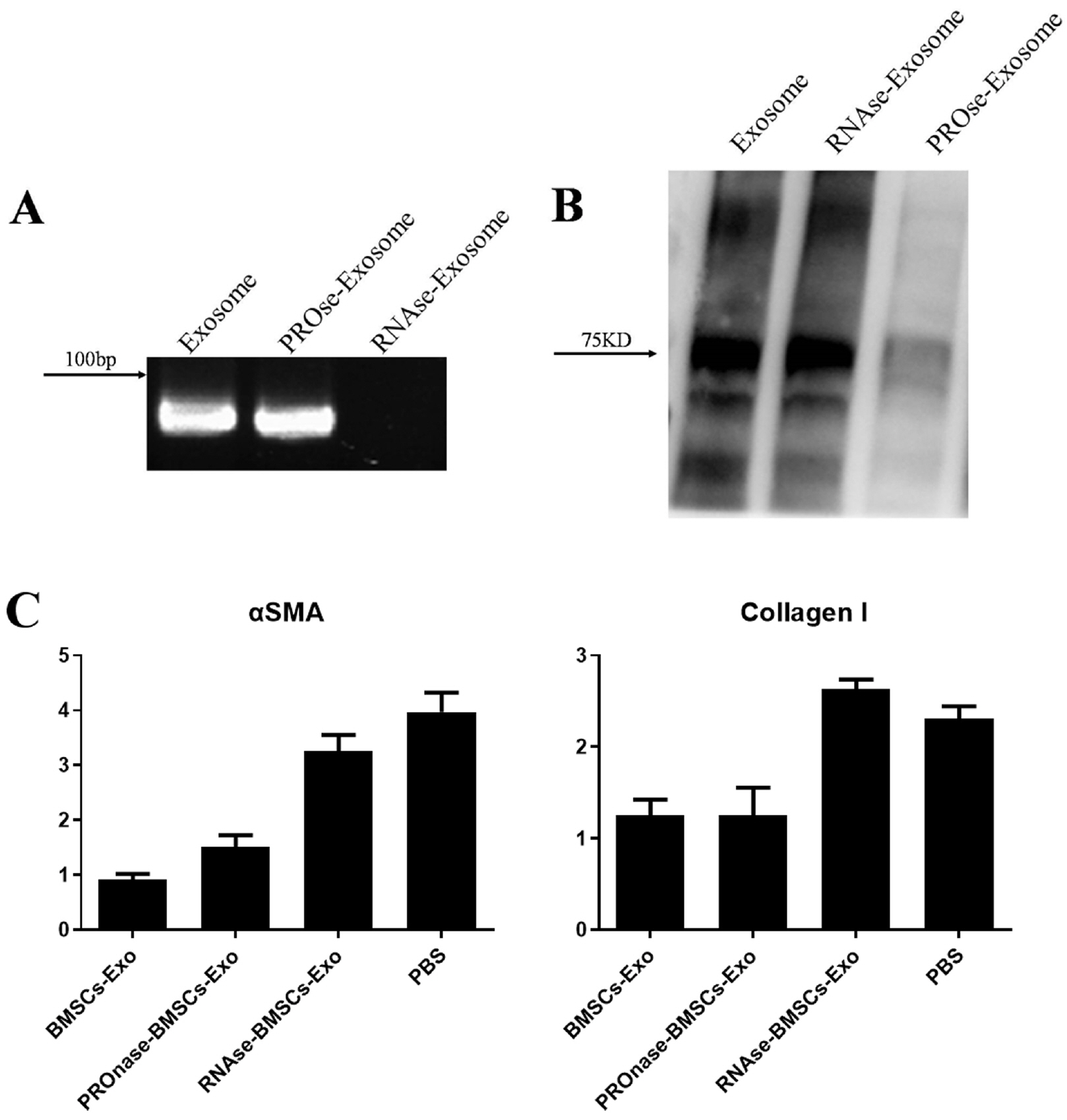
We used the magnetic bead affinity method to extract BMSC-Exo and analyzed its biological character. Then we co-cultured the BMSCs-Exo with endometrial cells to detect its effect. We injected BMSCs-Exo into the IUA mouse model. We over-expressed miR-29a in BMSCs-Exo by transient transfection, then used RT-PCR to analyze the expression of the related genes.
Results
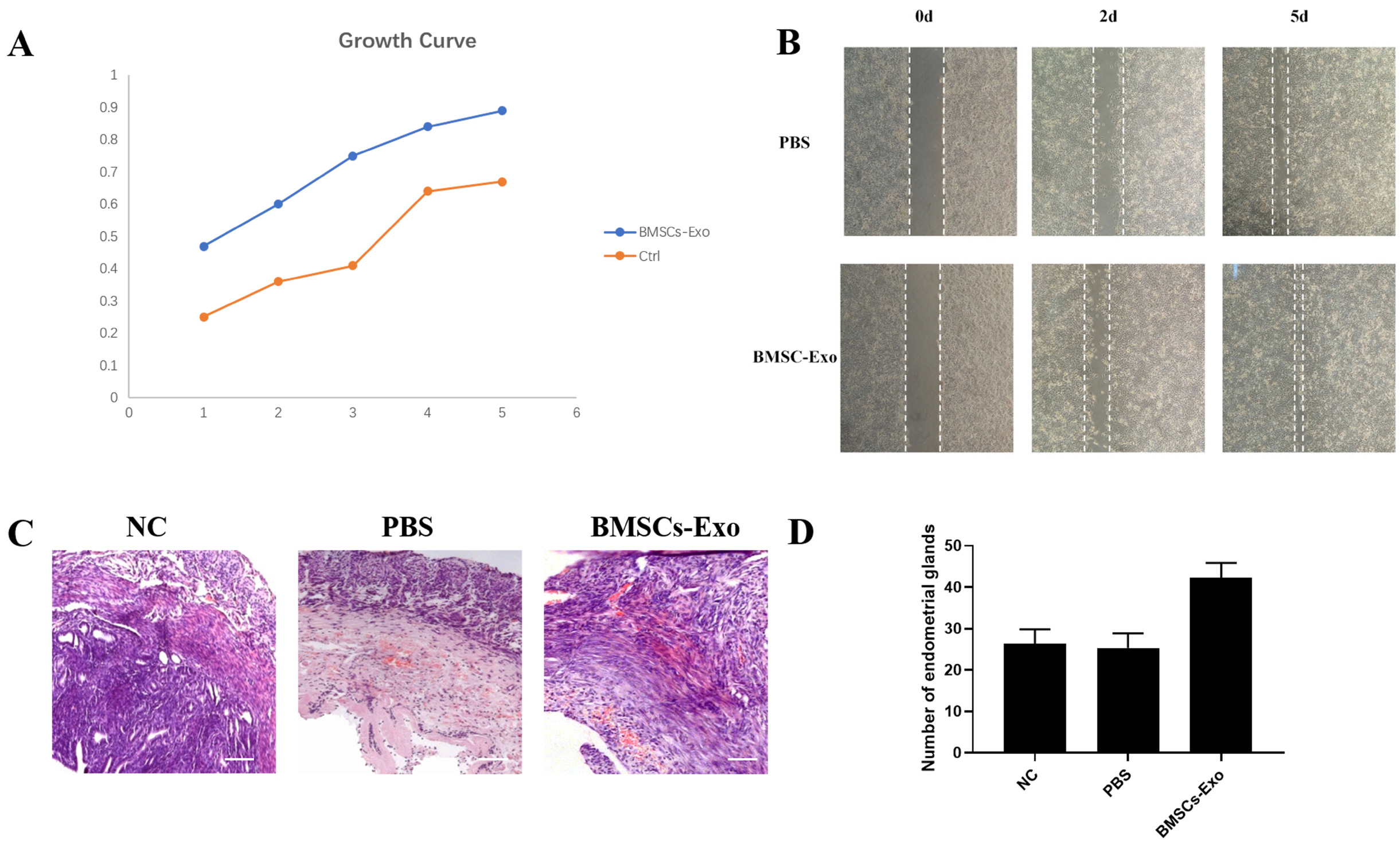
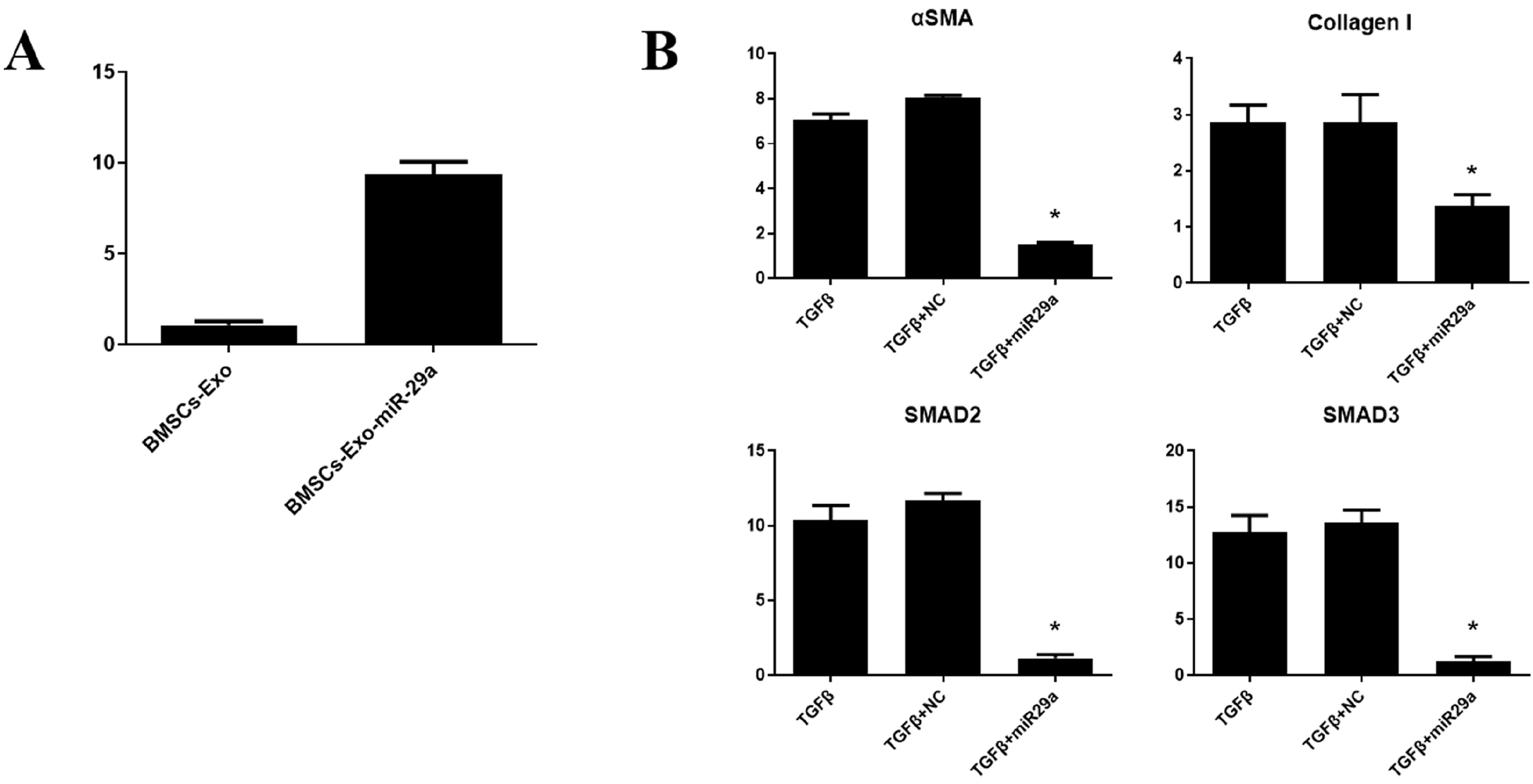
BMSCs-Exo expressed exosome-specific proteins CD9, CD63, and CD81. BMSCs-Exo could bring the contents into the target cells. BMSCs-Exo can promote endometrial repair in vitro or in vivo. BMSCs-Exo overexpressing miR-29a can reduce αSMA, Collagen I, SMAD2, and SMAD3.
Conclusions
In this study, we successfully isolated BMSCs-Exo and proved its character and biological activity. BMSCs-Exo can promote cell proliferation and cell migration in vitro and can repair damaged endometrium in the IUA model. The presence of miR-29a in BMSCs-Exo may be an important factor in its resistance to fibrosis during endometrial repair of IUA. This study provides new ideas for the treatment of patients with IUA and has important clinical research significance.
Figure
Cited by 1 articles
-
Extracellular Vesicles Derived from Mesenchymal Stem Cells as Cell-Free Therapy for Intrauterine Adhesion
Chao Li, Yuanjing Hu
Int J Stem Cells. 2023;16(3):260-268. doi: 10.15283/ijsc21177.
Reference
-
References
1. Nishi Y, Takeshita T. 2006; Asherman syndrome. Nihon Rinsho. Suppl 2:418–421. Japanese.2. March CM. 2011; Asherman's syndrome. Semin Reprod Med. 29:83–94. DOI: 10.1055/s-0031-1272470. PMID: 21437822.
Article3. Zikopoulos KA, Kolibianakis EM, Platteau P, de Munck L, Tournaye H, Devroey P, Camus M. 2004; Live delivery rates in subfertile women with Asherman's syndrome after hysteroscopic adhesiolysis using the resectoscope or the Versapoint system. Reprod Biomed Online. 8:720–725. DOI: 10.1016/S1472-6483(10)61654-9. PMID: 15169591.
Article4. Thomson AJ, Abbott JA, Deans R, Kingston A, Vancaillie TG. 2009; The management of intrauterine synechiae. Curr Opin Obstet Gynecol. 21:335–341. DOI: 10.1097/GCO.0b013e32832e07fc. PMID: 19550326.
Article5. Chen F, Duan H, Zhang Y, Wu YH. 2010; Effect and mechanism of formation of intrauterine adhesion at different dose of estrogen. Zhonghua Fu Chan Ke Za Zhi. 45:917–920. Chinese.6. Deans R, Abbott J. 2010; Review of intrauterine adhesions. J Minim Invasive Gynecol. 17:555–569. DOI: 10.1016/j.jmig.2010.04.016. PMID: 20656564.
Article7. Yu D, Wong YM, Cheong Y, Xia E, Li TC. 2008; Asherman syndrome--one century later. Fertil Steril. 89:759–779. DOI: 10.1016/j.fertnstert.2008.02.096. PMID: 18406834.
Article8. Morrison SJ, Uchida N, Weissman IL. 1995; The biology of hematopoietic stem cells. Annu Rev Cell Dev Biol. 11:35–71. DOI: 10.1146/annurev.cb.11.110195.000343. PMID: 8689561.
Article9. Bianco P, Riminucci M, Gronthos S, Robey PG. 2001; Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 19:180–192. DOI: 10.1634/stemcells.19-3-180. PMID: 11359943.
Article10. Alvarez-Buylla A, García-Verdugo JM, Tramontin AD. 2001; A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci. 2:287–293. DOI: 10.1038/35067582. PMID: 11283751.
Article11. Gallacher L, Murdoch B, Wu D, Karanu F, Fellows F, Bhatia M. 2000; Identification of novel circulating human embryonic blood stem cells. Blood. 96:1740–1747. DOI: 10.1182/blood.V96.5.1740. PMID: 10961872.
Article12. Du H, Taylor HS. 2007; Contribution of bone marrow-derived stem cells to endometrium and endometriosis. Stem Cells. 25:2082–2086. DOI: 10.1634/stemcells.2006-0828. PMID: 17464086.
Article13. Nagori CB, Panchal SY, Patel H. 2011; Endometrial regeneration using autologous adult stem cells followed by conception by in vitro fertilization in a patient of severe Asherman's syndrome. J Hum Reprod Sci. 4:43–48. DOI: 10.4103/0974-1208.82360. PMID: 21772740. PMCID: PMC3136069.
Article14. Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. 1987; Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 262:9412–9420. PMID: 3597417.
Article15. Ogawa Y, Kanai-Azuma M, Akimoto Y, Kawakami H, Yanoshita R. 2008; Exosome-like vesicles with dipeptidyl peptidase IV in human saliva. Biol Pharm Bull. 31:1059–1062. DOI: 10.1248/bpb.31.1059. PMID: 18520029.
Article16. Tkach M, Théry C. 2016; Communication by extracellular vesicles: where we are and where we need to go. Cell. 164:1226–1232. DOI: 10.1016/j.cell.2016.01.043. PMID: 26967288.
Article17. Raposo G, Stoorvogel W. 2013; Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 200:373–383. DOI: 10.1083/jcb.201211138. PMID: 23420871. PMCID: PMC3575529.
Article18. van Niel G, Raposo G, Candalh C, Boussac M, Hershberg R, Cerf-Bensussan N, Heyman M. 2001; Intestinal epithelial cells secrete exosome-like vesicles. Gastroenterology. 121:337–349. DOI: 10.1053/gast.2001.26263. PMID: 11487543.
Article19. Blanchard N, Lankar D, Faure F, Regnault A, Dumont C, Raposo G, Hivroz C. 2002; TCR activation of human T cells induces the production of exosomes bearing the TCR/CD3/zeta complex. J Immunol. 168:3235–3241. DOI: 10.4049/jimmunol.168.7.3235. PMID: 11907077.
Article20. Théry C, Amigorena S, Raposo G, Clayton A. 2006; Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 30:3.22.1–3.22.29. DOI: 10.1002/0471143030.cb0322s30. PMID: 18228490.
Article21. Conforti A, Alviggi C, Mollo A, De Placido G, Magos A. 2013; The management of Asherman syndrome: a review of literature. Reprod Biol Endocrinol. 11:118. DOI: 10.1186/1477-7827-11-118. PMID: 24373209. PMCID: PMC3880005.
Article22. Salazar CA, Isaacson K, Morris S. 2017; A comprehensive review of Asherman's syndrome: causes, symptoms and treatment options. Curr Opin Obstet Gynecol. 29:249–256. DOI: 10.1097/GCO.0000000000000378. PMID: 28582327.
Article23. Berman JM. 2008; Intrauterine adhesions. Semin Reprod Med. 26:349–355. DOI: 10.1055/s-0028-1082393. PMID: 18756412.
Article24. Lin HY, Wang FS, Yang YL, Huang YH. 2019; MicroRNA-29a suppresses CD36 to ameliorate high fat diet-induced steatohepatitis and liver fibrosis in mice. Cells. 8:1298. DOI: 10.3390/cells8101298. PMID: 31652636. PMCID: PMC6830328.
Article25. Harmanci D, Erkan EP, Kocak A, Akdogan GG. 2017; Role of the microRNA-29 family in fibrotic skin diseases. Biomed Rep. 6:599–604. DOI: 10.3892/br.2017.900. PMID: 28584629. PMCID: PMC5449962.
Article26. Huang YH, Yang YL, Wang FS. 2018; The role of miR-29a in the regulation, function, and signaling of liver fibrosis. Int J Mol Sci. 19:1889. DOI: 10.3390/ijms19071889. PMID: 29954104. PMCID: PMC6073598.
Article27. Timmers L, Lim SK, Arslan F, Armstrong JS, Hoefer IE, Doevendans PA, Piek JJ, El Oakley RM, Choo A, Lee CN, Pasterkamp G, de Kleijn DP. 2007; Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Res. 1:129–137. DOI: 10.1016/j.scr.2008.02.002. PMID: 19383393.
Article28. Hu J, Zhang L, Wang N, Ding R, Cui S, Zhu F, Xie Y, Sun X, Wu D, Hong Q, Li Q, Shi S, Liu X, Chen X. 2013; Mesenchymal stem cells attenuate ischemic acute kidney injury by inducing regulatory T cells through splenocyte interactions. Kidney Int. 84:521–531. DOI: 10.1038/ki.2013.114. PMID: 23615497. PMCID: PMC3778762.
Article29. Li T, Yan Y, Wang B, Qian H, Zhang X, Shen L, Wang M, Zhou Y, Zhu W, Li W, Xu W. 2013; Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev. 22:845–854. DOI: 10.1089/scd.2012.0395. PMID: 23002959. PMCID: PMC3585469.
Article30. Blazquez R, Sanchez-Margallo FM, de la Rosa O, Dalemans W, Alvarez V, Tarazona R, Casado JG. 2014; Immunomodulatory potential of human adipose mesenchymal stem cells derived exosomes on in vitro stimulated T cells. Front Immunol. 5:556. DOI: 10.3389/fimmu.2014.00556. PMID: 25414703. PMCID: PMC4220146.
Article31. Kim DK, Nishida H, An SY, Shetty AK, Bartosh TJ, Prockop DJ. 2016; Chromatographically isolated CD63+CD81+ extracellular vesicles from mesenchymal stromal cells rescue cognitive impairments after TBI. Proc Natl Acad Sci U S A. 113:170–175. DOI: 10.1073/pnas.1522297113. PMID: 26699510. PMCID: PMC4711859.
Article32. Pohlers D, Brenmoehl J, Löffler I, Müller CK, Leipner C, Schultze-Mosgau S, Stallmach A, Kinne RW, Wolf G. 2009; TGF-beta and fibrosis in different organs-molecular pathway imprints. Biochim Biophys Acta. 1792:746–756. DOI: 10.1016/j.bbadis.2009.06.004. PMID: 19539753.33. Chen B, Li R, Yan N, Chen G, Qian W, Jiang HL, Ji C, Bi ZG. 2015; Astragaloside IV controls collagen reduction in photoaging skin by improving transforming growth factor-β/Smad signaling suppression and inhibiting matrix metalloproteinase-1. Mol Med Rep. 11:3344–3348. DOI: 10.3892/mmr.2015.3212. PMID: 25591734. PMCID: PMC4368092.
Article34. Inagaki Y, Kushida M, Higashi K, Itoh J, Higashiyama R, Hong YY, Kawada N, Namikawa K, Kiyama H, Bou-Gharios G, Watanabe T, Okazaki I, Ikeda K. 2005; Cell type-specific intervention of transforming growth factor beta/Smad signaling suppresses collagen gene expression and hepatic fibrosis in mice. Gastroenterology. 129:259–268. DOI: 10.1053/j.gastro.2005.03.088. PMID: 16012952.
Article35. Xiao J, Meng XM, Huang XR, Chung AC, Feng YL, Hui DS, Yu CM, Sung JJ, Lan HY. 2012; miR-29 inhibits bleomycin-induced pulmonary fibrosis in mice. Mol Ther. 20:1251–1260. DOI: 10.1038/mt.2012.36. PMID: 22395530. PMCID: PMC3369297.
Article36. van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, Hill JA, Olson EN. 2008; Dysre-gulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci U S A. 105:13027–13032. DOI: 10.1073/pnas.0805038105. PMID: 18723672. PMCID: PMC2529064.
Article37. Alizadeh M, Safarzadeh A, Beyranvand F, Ahmadpour F, Hajiasgharzadeh K, Baghbanzadeh A, Baradaran B. 2019; The potential role of miR-29 in health and cancer diagnosis, prognosis, and therapy. J Cell Physiol. 234:19280–19297. DOI: 10.1002/jcp.28607. PMID: 30950056.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- MiR-29a-3p Inhibits Proliferation and Osteogenic Differentiation of Human Bone Marrow Mesenchymal Stem Cells via Targeting FOXO3 and Repressing Wnt/β-Catenin Signaling in Steroid-Associated Osteonecrosis
- Extracellular Vesicles Derived from Mesenchymal Stem Cells as Cell-Free Therapy for Intrauterine Adhesion
- MiR-182-5p Mediated by Exosomes Derived From Bone Marrow Mesenchymal Stem Cell Attenuates Inflammatory Responses by Targeting TLR4 in a Mouse Model of Myocardial Infraction
- Exosomes from Tension Force-Applied Periodontal Ligament Cells Promote Mesenchymal Stem Cell Recruitment by Altering microRNA Profiles
- Intra-Articular Injection of miR-29a-3p of BMSCs Promotes Cartilage Self-Repairing and Alleviates Pain in the Rat Osteoarthritis