Korean J Orthod.
2020 Nov;50(6):391-400. 10.4041/kjod.2020.50.6.391.
Antifibrotic effects of sulforaphane treatment on gingival elasticity reduces orthodontic relapse after rotational tooth movement in beagle dogs
- Affiliations
-
- 1Department of Orthodontics, Institute of Craniofacial Deformity, Yonsei University College of Dentistry, Seoul, Korea
- 2Department of Oral Pathology, Oral Cancer Research Institute, Yonsei University College of Dentistry, Seoul, Korea
- 3Division of Anatomy and Developmental Biology, Department of Oral Biology, Yonsei University College of Dentistry, Seoul, Korea
- KMID: 2508447
- DOI: http://doi.org/10.4041/kjod.2020.50.6.391
Abstract
Objective
Increased gingival elasticity has been implicated as the cause of relapse following orthodontic rotational tooth movement and approaches to reduce relapse are limited. This study aimed to investigate the effects of sulforaphane (SFN), an inhibitor of osteoclastogenesis, on gene expression in gingival fibroblasts and relapse after rotational tooth movement in beagle dogs.
Methods
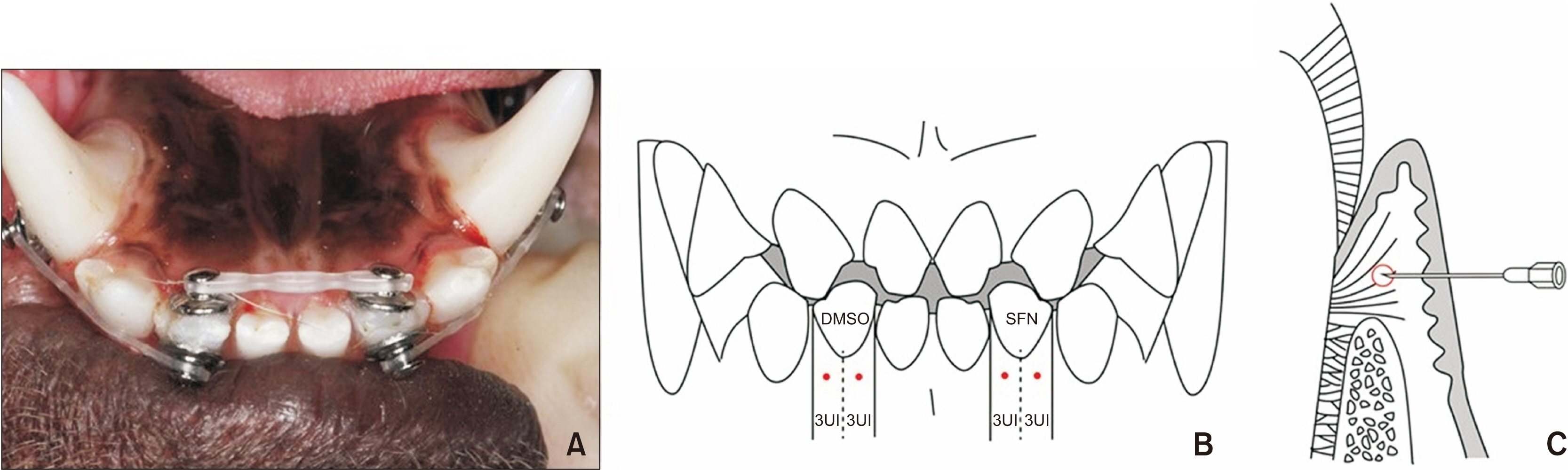
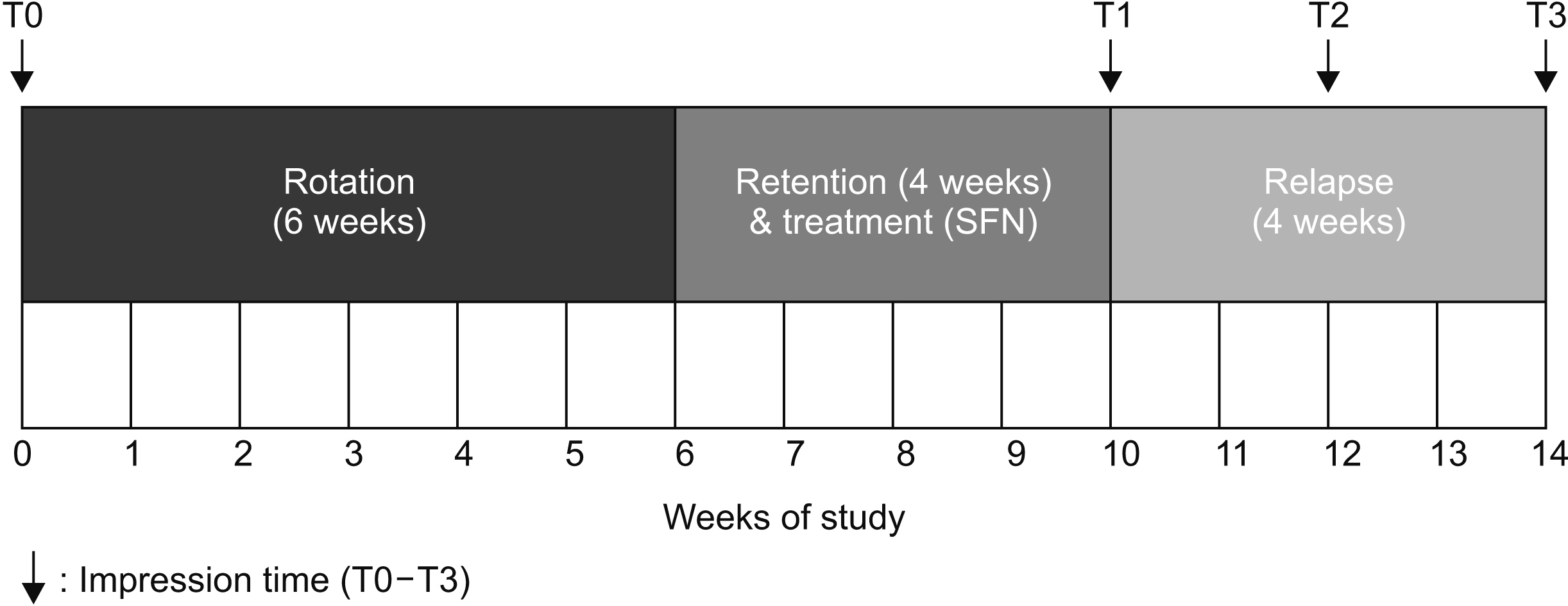
The lower lateral incisors of five beagle dogs were rotated. SFN or dimethylsulfoxide (DMSO) were injected into the supra-alveolar gingiva of the experimental and control group, respectively, and the effect of SFN on relapse tendency was evaluated. Changes in mRNA expression of extracellular matrix components associated with gingival elasticity in beagles were investigated by real-time polymerase chain reaction. Morphology and arrangement of collagen fibers were observed on Masson’s trichrome staining of buccal gingival tissues of experimental and control teeth.
Results
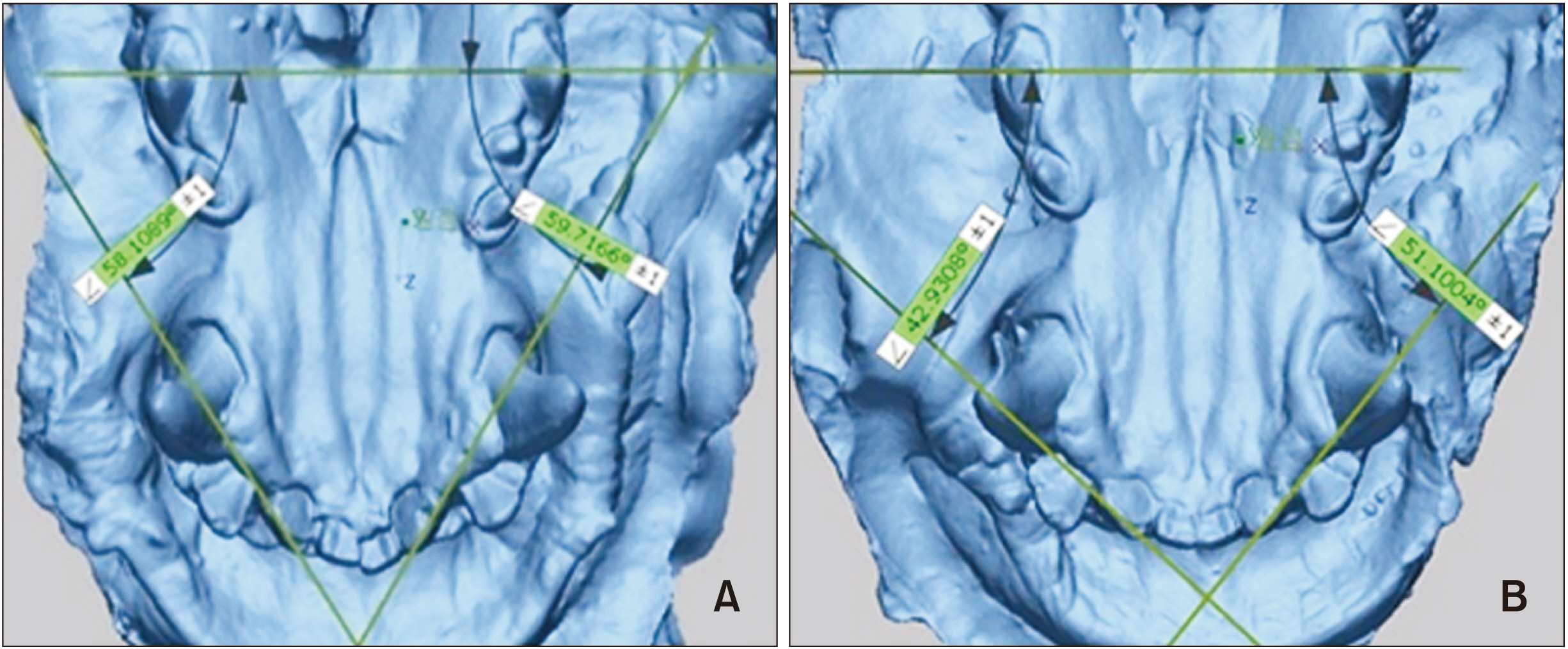
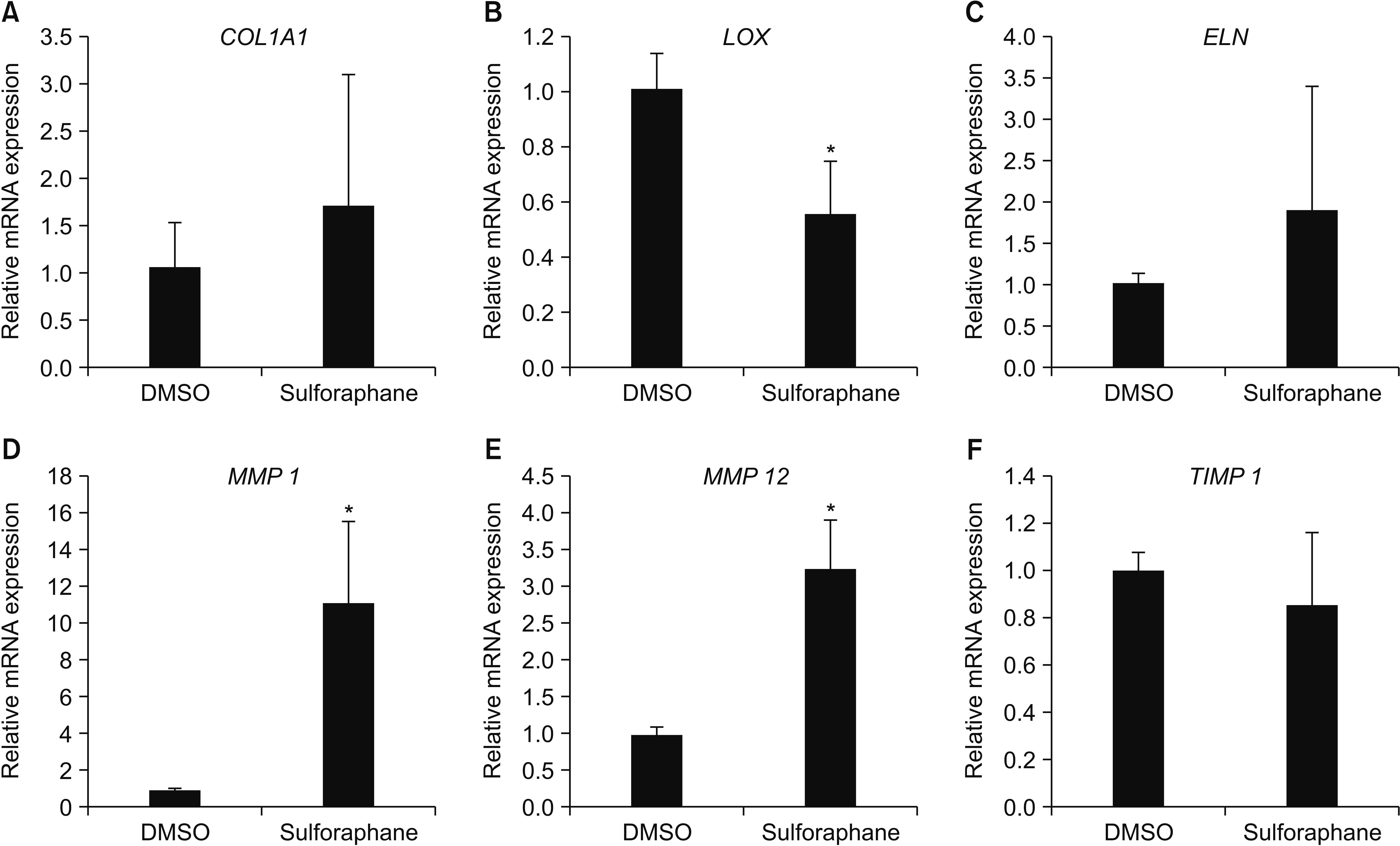
SFN reduced the amount and percentage of relapse of orthodontic rotation. It also decreased the gene expression of lysyl oxidase and increased the gene expression of matrix metalloproteinase (MMP) 1 and MMP 12, compared with DMSO control subjects. Histologically, collagen fiber bundles were arranged irregularly and were not well connected in the SFN-treated group, whereas the fibers extended in parallel and perpendicular directions toward the gingiva and alveolar bone in a more regular and well-ordered arrangement in the DMSO-treated group.
Conclusions
Our findings demonstrated that SFN treatment may be a promising pharmacologic approach to prevent orthodontic rotational relapse caused by increased gingival elasticity of rotated teeth in beagle dogs.
Keyword
Figure
Reference
-
1. Reitan K. 1959; Tissue rearrangement during retention of orthodontically rotated teeth. Angle Orthod. 29:105–13.2. Edwards JG. 1968; A study of the periodontium during orthodontic rotation of teeth. Am J Orthod. 54:441–61. DOI: 10.1016/0002-9416(68)90199-1. PMID: 5239302.
Article3. Edwards JG. 1970; A surgical procedure to eliminate rotational relapse. Am J Orthod. 57:35–46. DOI: 10.1016/0002-9416(70)90203-4. PMID: 5262003.
Article4. Kaplan RG. 1976; Clinical experiences with circumferential supracrestal fiberotomy. Am J Orthod. 70:147–53. DOI: 10.1016/S0002-9416(76)90315-8. PMID: 1066051.
Article5. Edwards JG. 1988; A long-term prospective evaluation of the circumferential supracrestal fiberotomy in alleviating orthodontic relapse. Am J Orthod Dentofacial Orthop. 93:380–7. DOI: 10.1016/0889-5406(88)90096-0. PMID: 3163217.
Article6. Redlich M, Rahamim E, Gaft A, Shoshan S. 1996; The response of supraalveolar gingival collagen to orthodontic rotation movement in dogs. Am J Orthod Dentofacial Orthop. 110:247–55. DOI: 10.1016/S0889-5406(96)80007-2. PMID: 8814024.
Article7. Ronnerman A, Thilander B, Heyden G. 1980; Gingival tissue reactions to orthodontic closure of extraction sites: histologic and histochemical studies. Am J Orthod. 77:620–5. DOI: 10.1016/0002-9416(80)90153-0. PMID: 6930162.8. Franchi M, D'Aloya U, De Pasquale V, Caldini E, Graziani E, Borea G, et al. 1989; Ultrastructural changes of collagen and elastin in human gingiva during orthodontic tooth movement. Bull Group Int Rech Sci Stomatol Odontol. 32:139–43. PMID: 2620139.9. Bumann A, Carvalho RS, Schwarzer CL, Yen EH. 1997; Collagen synthesis from human PDL cells following orthodontic tooth movement. Eur J Orthod. 19:29–37. DOI: 10.1093/ejo/19.1.29. PMID: 9071043.
Article10. Redlich M, Palmon A, Zaks B, Geremi E, Rayzman S, Shoshan S. 1998; The effect of centrifugal force on the transcription levels of collagen type I and collagenase in cultured canine gingival fibroblasts. Arch Oral Biol. 43:313–6. DOI: 10.1016/S0003-9969(97)00108-8. PMID: 9839707.
Article11. Redlich M, Asher Roos H, Reichenberg E, Zaks B, Mussig D, Baumert U, et al. 2004; Expression of tropoelastin in human periodontal ligament fibroblasts after simulation of orthodontic force. Arch Oral Biol. 49:119–24. DOI: 10.1016/j.archoralbio.2003.08.002. PMID: 14693205.
Article12. Takano M, Yamaguchi M, Nakajima R, Fujita S, Kojima T, Kasai K. 2009; Effects of relaxin on collagen type I released by stretched human periodontal ligament cells. Orthod Craniofac Res. 12:282–8. DOI: 10.1111/j.1601-6343.2009.01463.x. PMID: 19840280.
Article13. Hirate Y, Yamaguchi M, Kasai K. 2012; Effects of relaxin on relapse and periodontal tissue remodeling after experimental tooth movement in rats. Connect Tissue Res. 53:207–19. DOI: 10.3109/03008207.2011.628060. PMID: 22141456.
Article14. Kanzaki H, Shinohara F, Itohiya-Kasuya K, Ishikawa M, Nakamura Y. 2015; Nrf2 activation attenuates both orthodontic tooth movement and relapse. J Dent Res. 94:787–94. DOI: 10.1177/0022034515577814. PMID: 25795629.
Article15. Alam J, Stewart D, Touchard C, Boinapally S, Choi AM, Cook JL. 1999; Nrf2, a Cap'n'Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J Biol Chem. 274:26071–8. DOI: 10.1074/jbc.274.37.26071. PMID: 10473555.
Article16. Kanzaki H, Shinohara F, Kajiya M, Kodama T. 2013; The Keap1/Nrf2 protein axis plays a role in osteoclast differentiation by regulating intracellular reactive oxygen species signaling. J Biol Chem. 288:23009–20. DOI: 10.1074/jbc.M113.478545. PMID: 23801334. PMCID: PMC3743476.
Article17. Demirovic D, Rattan SI. 2011; Curcumin induces stress response and hormetically modulates wound healing ability of human skin fibroblasts undergoing ageing in vitro. Biogerontology. 12:437–44. DOI: 10.1007/s10522-011-9326-7. PMID: 21380847.
Article18. Hayashi R, Himori N, Taguchi K, Ishikawa Y, Uesugi K, Ito M, et al. 2013; The role of the Nrf2-mediated defense system in corneal epithelial wound healing. Free Radic Biol Med. 61:333–42. DOI: 10.1016/j.freeradbiomed.2013.04.008. PMID: 23587556.
Article19. Artaud-Macari E, Goven D, Brayer S, Hamimi A, Besnard V, Marchal-Somme J, et al. 2013; Nuclear factor erythroid 2-related factor 2 nuclear translocation induces myofibroblastic dedifferentiation in idiopathic pulmonary fibrosis. Antioxid Redox Signal. 18:66–79. DOI: 10.1089/ars.2011.4240. PMID: 22703534.
Article20. Kawarazaki A, Horinaka M, Yasuda S, Numajiri T, Nishino K, Sakai T. 2017; Sulforaphane suppresses cell growth and collagen expression of keloid fibroblasts. Wound Repair Regen. 25:224–33. DOI: 10.1111/wrr.12512. PMID: 28120534.
Article21. Kim SJ, Kang YG, Park JH, Kim EC, Park YG. 2013; Effects of low-intensity laser therapy on periodontal tissue remodeling during relapse and retention of orthodontically moved teeth. Lasers Med Sci. 28:325–33. DOI: 10.1007/s10103-012-1146-8. PMID: 22814894.
Article22. Svoboda EL, Shiga A, Deporter DA. 1981; A stereologic analysis of collagen phagocytosis by fibroblasts in three soft connective tissues with differing rates of collagen turnover. Anat Rec. 199:473–80. DOI: 10.1002/ar.1091990404. PMID: 7270910.
Article23. Lefevre M, Rucker RB. 1980; Aorta elastin turnover in normal and hypercholesterolemic Japanese quail. Biochim Biophys Acta. 630:519–29. DOI: 10.1016/0304-4165(80)90006-9. PMID: 6772235.
Article24. Shapiro SD, Endicott SK, Province MA, Pierce JA, Campbell EJ. 1991; Marked longevity of human lung parenchymal elastic fibers deduced from prevalence of D-aspartate and nuclear weapons-related radiocarbon. J Clin Invest. 87:1828–34. DOI: 10.1172/JCI115204. PMID: 2022748. PMCID: PMC295305.
Article25. Meng M, Lv C, Yang Q, He S, Wu S, Liu Y, et al. 2018; Expression of proteins of elastic fibers and collagen type I in orthodontically rotated teeth in rats. Am J Orthod Dentofacial Orthop. 154:249–59. DOI: 10.1016/j.ajodo.2017.11.030. PMID: 30075927.
Article26. Reiser K, McCormick RJ, Rucker RB. 1992; Enzymatic and nonenzymatic cross-linking of collagen and elastin. FASEB J. 7:2439–49. DOI: 10.1096/fasebj.6.7.1348714. PMID: 1348714.
Article27. Niewoehner DE, Hoidal JR. 1982; Lung fibrosis and emphysema: divergent responses to a common injury? Science. 217:359–60. DOI: 10.1126/science.7089570. PMID: 7089570.
Article28. Ma RH, Tsai CC, Shieh TY. 1995; Increased lysyl oxidase activity in fibroblasts cultured from oral submucous fibrosis associated with betel nut chewing in Taiwan. J Oral Pathol Med. 24:407–12. DOI: 10.1111/j.1600-0714.1995.tb01210.x. PMID: 8537914.
Article29. Van Doren SR. 2015; Matrix metalloproteinase interactions with collagen and elastin. Matrix Biol. 44-46:224–31. DOI: 10.1016/j.matbio.2015.01.005. PMID: 25599938. PMCID: PMC4466143.
Article30. Skjøt-Arkil H, Clausen RE, Nguyen QH, Wang Y, Zheng Q, Martinez FJ, et al. 2012; Measurement of MMP-9 and -12 degraded elastin (ELM) provides unique information on lung tissue degradation. BMC Pulm Med. 12:34. DOI: 10.1186/1471-2466-12-34. PMID: 22818364. PMCID: PMC3515477.
Article31. Yamada H, Sato T, Fukayama H. 2011; Diffusion patterns of anesthetic solution in the infiltration anesthesia depending on injection sites in the mandible of rats. Oral Sci Int. 8:50–4. DOI: 10.1016/S1348-8643(11)00026-7.
Article32. Cantu R, Steffe JA. Maxey L, Magnusson J, editors. 2013. Soft tissue healing considerations after surgery. Rehabilitation for the postsurgical orthopedic patient. 3rd ed. Elsevier Mosby;Louis, Mo: p. 15–25. DOI: 10.1016/B978-0-323-07747-7.00002-2.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Mode of tooth movement according to the timing of orthodontic force application after extraction
- Interleukin-1beta levels in human gingival crevicular fluid during orthodontic tooth movement
- Gingival recession of lower anterior incisors in orthodontic treatment
- Accelerated orthodontic tooth movement: surgical techniques and the regional acceleratory phenomenon
- The effects of ipriflavone on the periodontal reorganization following experimental tooth movement in the rat