Ann Surg Treat Res.
2020 Nov;99(5):294-304. 10.4174/astr.2020.99.5.294.
Refined surgical techniques to improve the patency of cryopreserved iliac artery homografts for middle hepatic vein reconstruction during living donor liver transplantation
- Affiliations
-
- 1Division of Liver Transplantation and Hepatobiliary Surgery, Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2508327
- DOI: http://doi.org/10.4174/astr.2020.99.5.294
Abstract
- Purpose
A cryopreserved iliac artery homograft (IAH) has not been considered suitable for middle hepatic vein (MHV) reconstruction during living donor liver transplantation (LDLT), primarily due to the low patency from its small diameter. We revised our surgical techniques for MHV reconstruction using an IAH to improve its patency.
Methods
This study analyzed the causes of early conduit occlusion and developed revised techniques to address this that had clinical application.
Results
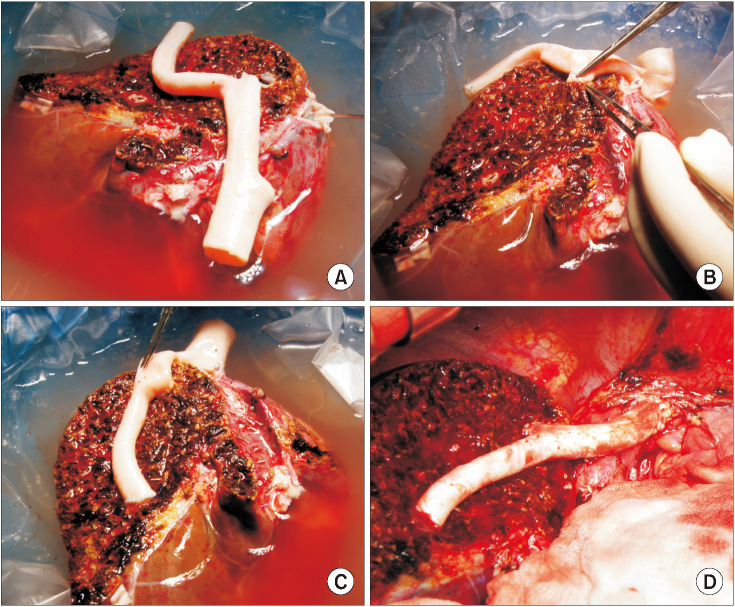
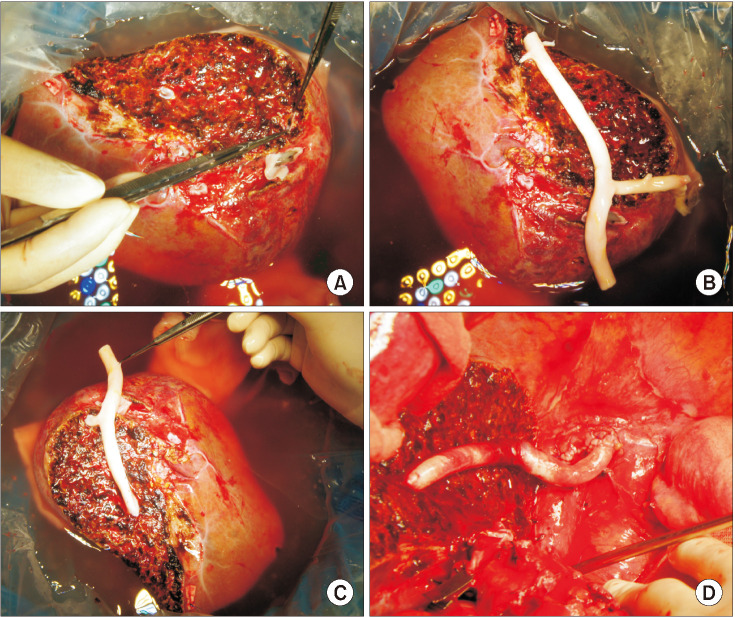
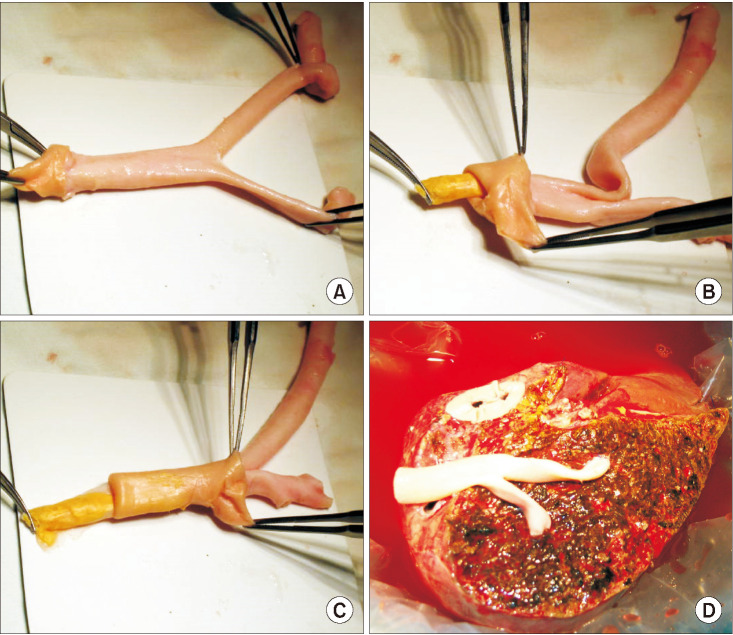
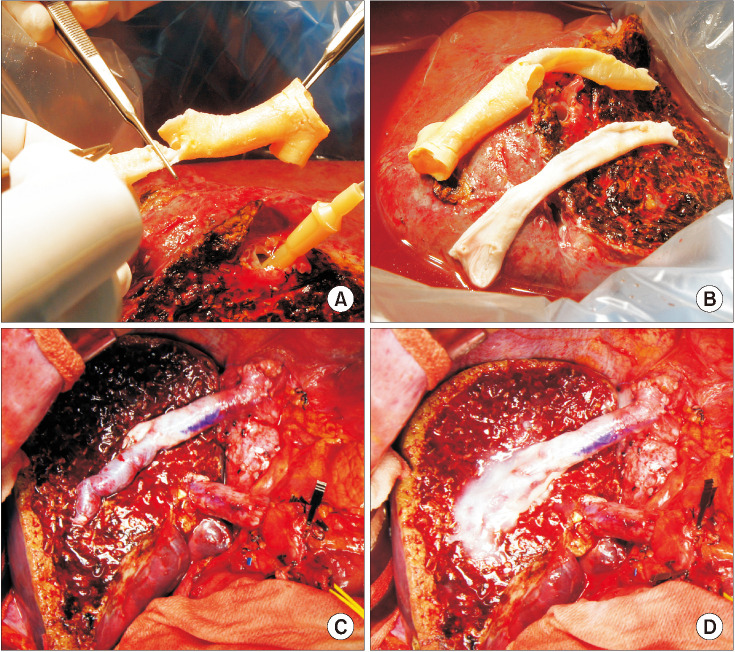
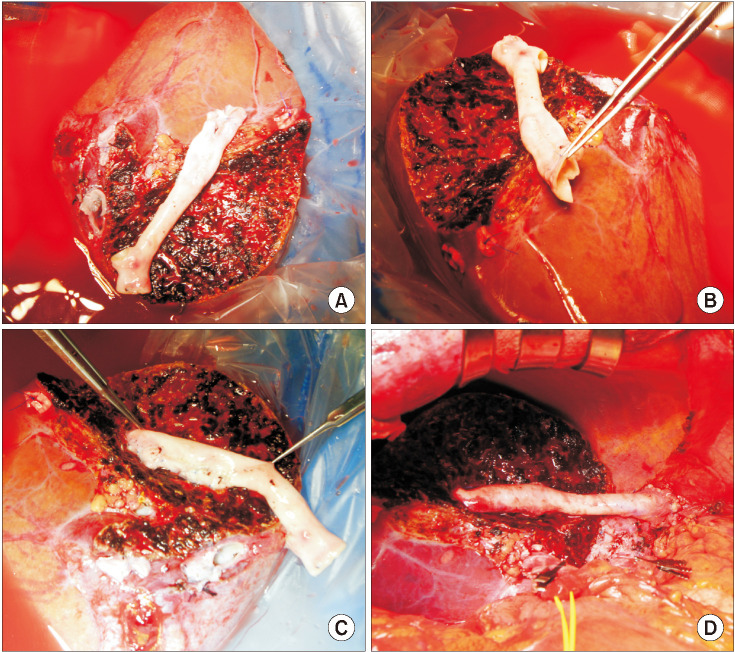
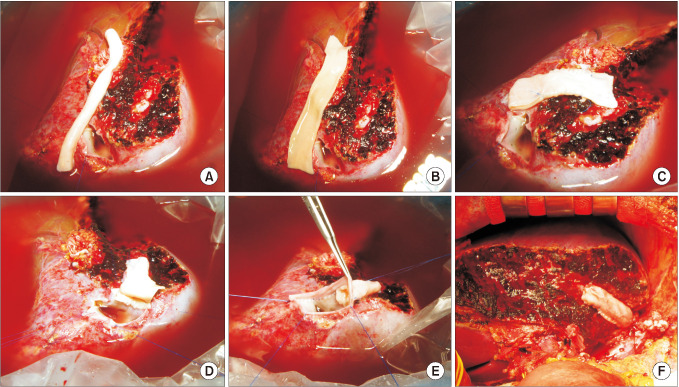
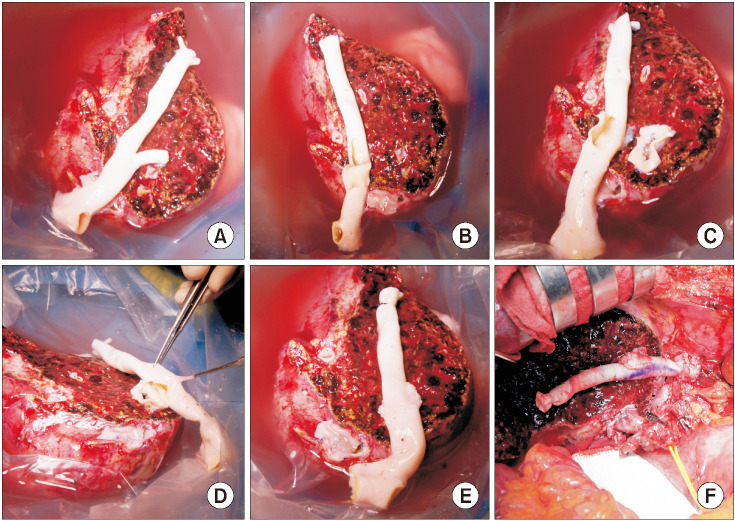
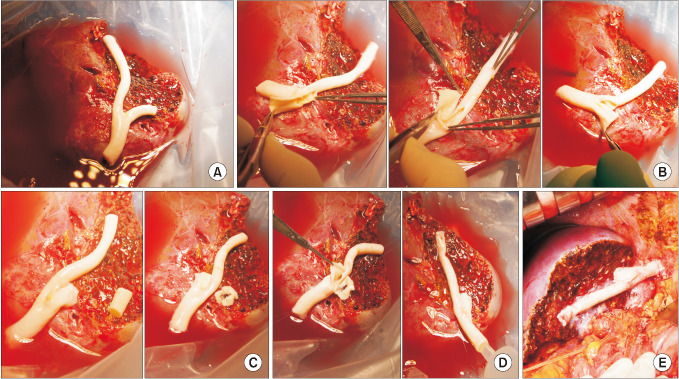
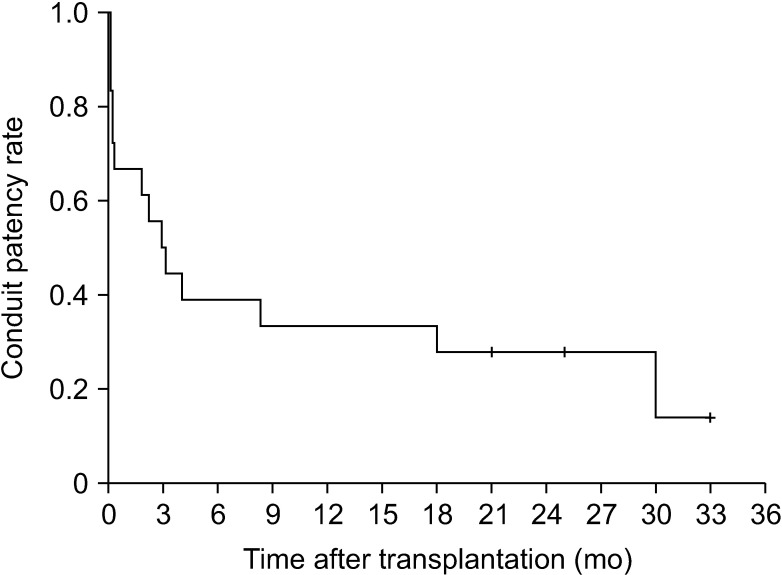
The potential risk factors for early conduit occlusion were the small IAH size, small graft in the segment V vein (V5) and segment VIII vein (V8) opening, and small recipient MHV-left hepatic vein stump. These factors were reflected to our revised surgical methods which included endarterectomy of the atherosclerotic plaque, unification of the internal and external iliac artery branches for large V5, and branch-patch arterioplasty for large V8. IAH endarterectomy, branch unification technique, and branch-patch arterioplasty were applied to 8, 5, and 5 patients, respectively and resulted in 1-month occlusion rates of 37.5%, 20.0%, and 40.0%, respectively. The overall patency rates of the IAH-MHV conduits in our 18 patients were 66.7% at 1 month, 38.9% at 3 months, and 33.3% at 1 year.
Conclusion
Our refined MHV reconstruction using an IAH improved short-term MHV conduit patency, but did not effectively prevent early conduit occlusion, particularly with a small- or medium-sized IAH. Individualized reconstruction designs during LDLT operation are needed when an IAH is used for a modified right liver graft.
Figure
Reference
-
1. Hwang S, Lee SG, Lee YJ, Sung KB, Park KM, Kim KH, et al. Lessons learned from 1,000 living donor liver transplantations in a single center: how to make living donations safe. Liver Transpl. 2006; 12:920–927. PMID: 16721780.2. Hwang S, Lee SG, Ahn CS, Park KM, Kim KH, Moon DB, et al. Cryopreserved iliac artery is indispensable interposition graft material for middle hepatic vein reconstruction of right liver grafts. Liver Transpl. 2005; 11:644–649. PMID: 15915499.3. Sugawara Y, Makuuchi M, Akamatsu N, Kishi Y, Niiya T, Kaneko J, et al. Refinement of venous reconstruction using cryopreserved veins in right liver grafts. Liver Transpl. 2004; 10:541–547. PMID: 15048798.4. Hwang S, Lee SG, Song GW, Lee HJ, Park JI, Ryu JH. Use of endarterectomized atherosclerotic artery allograft for hepatic vein reconstruction of living donor right lobe graft. Liver Transpl. 2007; 13:306–308. PMID: 17256786.5. Hwang S, Jung DH, Ha TY, Ahn CS, Moon DB, Kim KH, et al. Usability of ringed polytetrafluoroethylene grafts for middle hepatic vein reconstruction during living donor liver transplantation. Liver Transpl. 2012; 18:955–965. PMID: 22511404.6. Kim SH, Hwang S, Ha TY, Song GW, Jung DH, Ahn CS, et al. Usability of cryopreserved aortic allografts for middle hepatic vein reconstruction during living-donor liver transplantation. J Gastrointest Surg. 2016; 20:1049–1055. PMID: 26666546.7. Park GC, Hwang S, Ha TY, Song GW, Jung DH, Ahn CS, et al. Hemashield vascular graft is a preferable prosthetic graft for middle hepatic vein reconstruction in living donor liver transplantation. Ann Transplant. 2019; 24:639–646. PMID: 31844037.8. Ha TY, Hwang S, Jung DH, Ahn CS, Kim KH, Moon DB, et al. Complications analysis of polytetrafluoroethylene grafts used for middle hepatic vein reconstruction in living-donor liver transplantation. Transplant Proc. 2014; 46:845–849. PMID: 24767363.9. Hsu SC, Thorat A, Yang HR, Poon KS, Li PC, Yeh CC, et al. Assessing the safety of expanded polytetrafluoroethylene synthetic grafts in living donor liver transplantation: graft migration into hollow viscous organs. Diagnosis and treatment options. Med Sci Monit. 2017; 23:3284–3292. PMID: 28683053.10. Koc C, Akbulut S, Ozdemir F, Kose A, Isik B, Yologlu S, et al. Analysis of risk factors affecting the development of infection in artificial vascular grafts used for reconstruction of middle hepatic vein tributaries in living donor liver transplantation. Transplantation. 2019; 103:1871–1876. PMID: 30747841.11. Chung MH, Chuang CC, Liaw LF, Chen CY, Chen IM, Hsu CP, et al. Thrombotic ringed polytetrafluoroethylene graft with infection after living-donor liver transplantation. Transplant Proc. 2018; 50:2606–2610. PMID: 30401360.12. Ko GY, Sung KB, Yoon HK, Kim JH, Song HY, Seo TS, et al. Endovascular treatment of hepatic venous outflow obstruction after living-donor liver transplantation. J Vasc Interv Radiol. 2002; 13:591–599. PMID: 12050299.13. Ko GY, Sung KB, Yoon HK, Kim KR, Kim JH, Gwon DI, et al. Early posttransplant hepatic venous outflow obstruction: longterm efficacy of primary stent placement. Liver Transpl. 2008; 14:1505–1511. PMID: 18825710.14. Kirchner VA, Hwang S, Song GW, Ahn CS, Moon DB, Kim KH, et al. Resolution of hepatic venous congestion following gradual occlusion of middle hepatic vein interposition graft in living donor liver transplantation. Ann Transplant. 2016; 21:619–625. PMID: 27713391.15. Hwang S, Lee SG, Park KM, Kim KH, Ahn CS, Lee YJ, et al. Hepatic venous congestion in living donor liver transplantation: preoperative quantitative prediction and follow-up using computed tomography. Liver Transpl. 2004; 10:763–770. PMID: 15162471.16. Ha TY, Kim YH, Chang JW, Park Y, Han Y, Kwon H, et al. Clinical outcomes of cryopreserved arterial allograft used as a vascular conduit for hemodialysis. J Korean Med Sci. 2016; 31:1266–1272. PMID: 27478338.17. Kwon H, Kwon H, Hong JP, Han Y, Park H, Song GW, et al. Use of cryopreserved cadaveric arterial allograft as a vascular conduit for peripheral arterial graft infection. Ann Surg Treat Res. 2015; 89:51–54. PMID: 26131446.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Refined surgical techniques to improve the patency of cryopreserved iliac artery homografts for middle hepatic vein reconstruction during living donor liver transplantation
- Human dermis as a new substitute for middle hepatic vein during living donor liver transplantation: early results from ongoing clinical trial
- Hepatic Artery Reconstruction Using the Right Gastroepiploic Artery for Hepatic Artery Inflow in a Living Donor Liver Transplantation
- Experience with Microsurgical Reconstruction of the Hepatic Artery in 100 Living Donor Liver Transplantation
- Revolution and Refinement of Surgical Techniques for Living Donor Partial Liver Transplantation