Cancer Res Treat.
2020 Oct;52(4):1041-1049. 10.4143/crt.2020.057.
Survival, Prognostic Factors, and Volumetric Analysis of Extent of Resection for Anaplastic Gliomas
- Affiliations
-
- 1Department of Neurosurgery, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea
- 2Department of Neurosurgery, Yonsei University College of Medicine, Seoul, Korea
- 3Department of Neurosurgery, Ajou University Hospital, Ajou University School of Medicine, Suwon, Korea
- 4Brain Tumor Center, Severance Hospital, Yonsei University Health System, Seoul, Korea
- 5Brain Research Institute, Yonsei University College of Medicine, Seoul, Korea
- 6Department of Pathology, Yonsei University College of Medicine, Seoul, Korea
- 7Department of Radiology, Yonsei University College of Medicine, Seoul, Korea
- 8Division of Oncology, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea
- 9Department of Radiation Oncology, Yonsei University College of Medicine, Seoul, Korea
- KMID: 2507931
- DOI: http://doi.org/10.4143/crt.2020.057
Abstract
- Purpose
The aim of this study is to evaluate the survival rate and prognostic factors of anaplastic gliomas according to the 2016 World Health Organization classification, including extent of resection (EOR) as measured by contrast-enhanced T1-weighted magnetic resonance imaging (MRI) and the T2-weighted MRI.
Materials and Methods
The records of 113 patients with anaplastic glioma who were newly diagnosed at our institute between 2000 and 2013 were retrospectively reviewed. There were 62 cases (54.9%) of anaplastic astrocytoma, isocitrate dehydrogenase (IDH) wild-type (AAw), 18 cases (16.0%) of anaplastic astrocytoma, IDH-mutant, and 33 cases (29.2%) of anaplastic oligodendroglioma, IDH-mutant and 1p/19q-codeleted.
Results
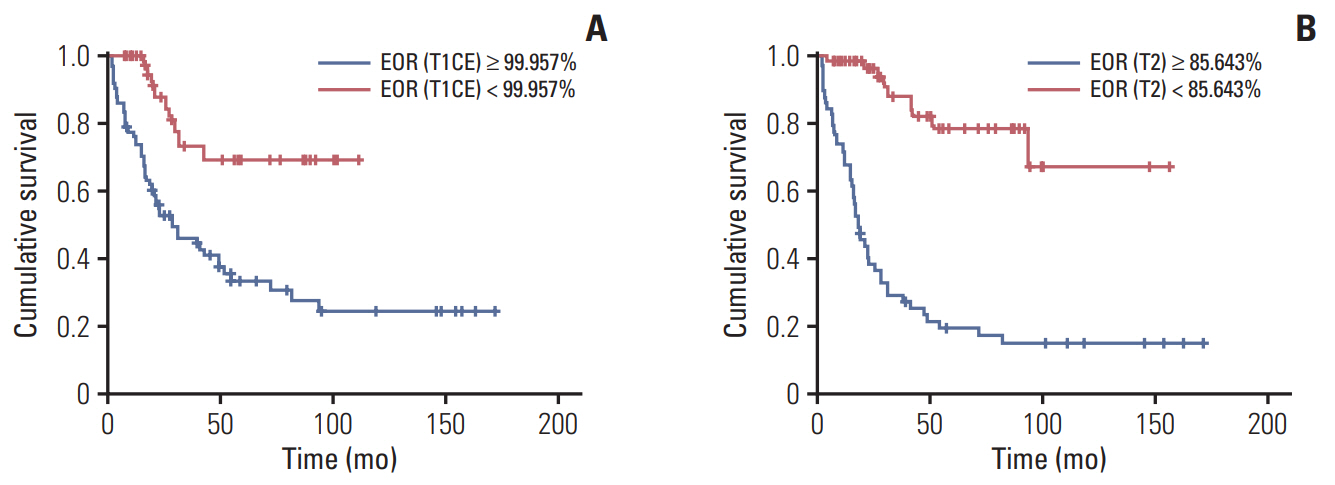
The median overall survival (OS) was 48.4 months in the whole anaplastic glioma group and 21.5 months in AAw group. In multivariate analysis, age, preoperative Karnofsky Performance Scale score, O6-methylguanine-DNA methyltransferase (MGMT) methylation status, postoperative tumor volume, and EOR measured from the T2 MRI sequence were significant prognostic factors. The EOR cut-off point for OS measured in contrast-enhanced T1-weighted MRI and T2-weighted MRI were 99.96% and 85.64%, respectively.
Conclusions
We found that complete resection of the contrast-enhanced portion (99.96%) and more than 85.64% resection of the non-enhanced portion of the tumor have prognostic impacts on patient survival from anaplastic glioma.
Keyword
Figure
Reference
-
References
1. Ostrom QT, Gittleman H, Fulop J, Liu M, Blanda R, Kromer C, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008-2012. Neuro Oncol. 2015; 17 Suppl 4:iv1–62.
Article2. Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016; 131:803–20.
Article3. Nuno M, Birch K, Mukherjee D, Sarmiento JM, Black KL, Patil CG. Survival and prognostic factors of anaplastic gliomas. Neurosurgery. 2013; 73:458–65.4. Rogne SG, Konglund A, Scheie D, Helseth E, Meling TR. Anaplastic astrocytomas: survival and prognostic factors in a surgical series. Acta Neurochir (Wien). 2014; 156:1053–61.
Article5. Contal C, O’Quigley J. An application of changepoint methods in studying the effect of age on survival in breast cancer. Comput Stat Data Anal. 1999; 30:253–70.
Article6. Cairncross G, Wang M, Shaw E, Jenkins R, Brachman D, Buckner J, et al. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol. 2013; 31:337–43.
Article7. Chaichana KL, Kosztowski T, Niranjan A, Olivi A, Weingart JD, Laterra J, et al. Prognostic significance of contrast-enhancing anaplastic astrocytomas in adults. J Neurosurg. 2010; 113:286–92.
Article8. van den Bent MJ, Brandes AA, Taphoorn MJ, Kros JM, Kouwenhoven MC, Delattre JY, et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol. 2013; 31:344–50.9. Wick W, Hartmann C, Engel C, Stoffels M, Felsberg J, Stockhammer F, et al. NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J Clin Oncol. 2009; 27:5874–80.
Article10. Wick W, Roth P, Hartmann C, Hau P, Nakamura M, Stockhammer F, et al. Long-term analysis of the NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with PCV or temozolomide. Neuro Oncol. 2016; 18:1529–37.
Article11. Hildebrand J, Gorlia T, Kros JM, Afra D, Frenay M, Omuro A, et al. Adjuvant dibromodulcitol and BCNU chemotherapy in anaplastic astrocytoma: results of a randomised European Organisation for Research and Treatment of Cancer phase III study (EORTC study 26882). Eur J Cancer. 2008; 44:1210–6.
Article12. Hartmann C, Hentschel B, Wick W, Capper D, Felsberg J, Simon M, et al. Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas. Acta Neuropathol. 2010; 120:707–18.
Article13. Paleologos NA, Merrell RT. Anaplastic glioma. Curr Treat Options Neurol. 2012; 14:381–90.
Article14. Stupp R, Reni M, Gatta G, Mazza E, Vecht C. Anaplastic astrocytoma in adults. Crit Rev Oncol Hematol. 2007; 63:72–80.
Article15. Wang Y, Wang K, Wang J, Li S, Ma J, Dai J, et al. Identifying the association between contrast enhancement pattern, surgical resection, and prognosis in anaplastic glioma patients. Neuroradiology. 2016; 58:367–74.
Article16. Claes A, Idema AJ, Wesseling P. Diffuse glioma growth: a guerilla war. Acta Neuropathol. 2007; 114:443–58.
Article17. Keles GE, Chang EF, Lamborn KR, Tihan T, Chang CJ, Chang SM, et al. Volumetric extent of resection and residual contrast enhancement on initial surgery as predictors of outcome in adult patients with hemispheric anaplastic astrocytoma. J Neurosurg. 2006; 105:34–40.
Article18. Li YM, Suki D, Hess K, Sawaya R. The influence of maximum safe resection of glioblastoma on survival in 1229 patients: can we do better than gross-total resection? J Neurosurg. 2016; 124:977–88.
Article19. McGirt MJ, Chaichana KL, Gathinji M, Attenello FJ, Than K, Olivi A, et al. Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J Neurosurg. 2009; 110:156–62.
Article20. Nitta M, Muragaki Y, Maruyama T, Ikuta S, Komori T, Maebayashi K, et al. Proposed therapeutic strategy for adult low-grade glioma based on aggressive tumor resection. Neurosurg Focus. 2015; 38:E7.
Article21. Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg. 2011; 115:3–8.
Article22. Smith JS, Chang EF, Lamborn KR, Chang SM, Prados MD, Cha S, et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol. 2008; 26:1338–45.
Article23. Devaux BC, O’Fallon JR, Kelly PJ. Resection, biopsy, and survival in malignant glial neoplasms: a retrospective study of clinical parameters, therapy, and outcome. J Neurosurg. 1993; 78:767–75.24. Tortosa A, Vinolas N, Villa S, Verger E, Gil JM, Brell M, et al. Prognostic implication of clinical, radiologic, and pathologic features in patients with anaplastic gliomas. Cancer. 2003; 97:1063–71.
Article25. Fujii Y, Muragaki Y, Maruyama T, Nitta M, Saito T, Ikuta S, et al. Threshold of the extent of resection for WHO grade III gliomas: retrospective volumetric analysis of 122 cases using intraoperative MRI. J Neurosurg. 2018; 129:1–9.
Article26. Pessina F, Navarria P, Cozzi L, Ascolese AM, Simonelli M, Santoro A, et al. Value of surgical resection in patients with newly diagnosed grade III glioma treated in a multimodal approach: surgery, chemotherapy and radiotherapy. Ann Surg Oncol. 2016; 23:3040–6.
Article27. Tanaka S, Meyer FB, Buckner JC, Uhm JH, Yan ES, Parney IF. Presentation, management, and outcome of elderly patients with newly-diagnosed anaplastic astrocytoma. J Neurooncol. 2012; 110:227–35.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anaplastic Meningioma: Clinical Characteristics, Prognostic Factors and Survival Outcome
- The Long-Term Survival in Patients with Anaplastic Astrocytoma or Glioblastoma
- The Study for Prognostic Factors in Anaplastic Carcinoma of the Thyroid
- Interim Tumor Progression and Volumetric Changes of Surgical Cavitiesduring the Surgery-to-Radiotherapy Interval in Anaplastic Gliomas:Implications for Additional Pre-radiotherapy Magnetic Resonance Imaging
- Expression of Trans forming Growth Factor-a and Proliferating Cell Nuclear Antigen in Human Gliomas