Infect Chemother.
2020 Mar;52(1):39-47. 10.3947/ic.2020.52.1.39.
The Rate of Acquisition of Carbapenemase-Producing Enterobacteriaceae among Close Contact Patients Depending on Carbapenemase Enzymes
- Affiliations
-
- 1Department of Infectious Diseases, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 2Office for Infection Control, Asan Medical Center, Seoul, Korea
- 3Department of Laboratory Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 4Division of Infectious Diseases, Department of Internal Medicine, Soonchunhyang University Cheonan Hospital, Soonchunhyang University College of Medicine, Cheoan, Korea
- KMID: 2507322
- DOI: http://doi.org/10.3947/ic.2020.52.1.39
Abstract
- Background
Carbapenemase-producing Enterobacteriaceae (CPE) are highly drug-resistant pathogens. Screening the contacts of newly-identified CPE patients is crucial for nosocomial transmission control. We evaluated the acquisition rate of CPE in close contacts as a function of CPE genotype.
Materials and Methods
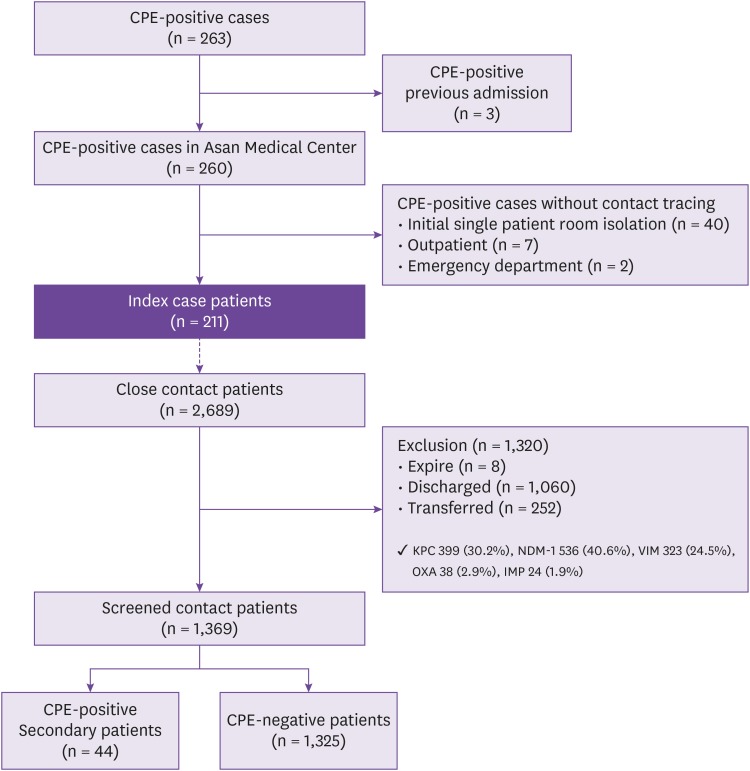
This study was conducted in Asan Medical Center, a 2,700-bed, tertiary teaching hospital in Seoul, Korea, between November 2010 and October 2017. Index cases were defined as patients with positive tests for CPE from any infected or colonized site during hospitalization who had no direct epidemiologic linkage with existing CPE patients; close contact patients were defined as those whose hospital stay overlapped with the stay of an index case for at least one day and who occupied the same room or intensive care unit (ICU). Secondary patients were defined as those who produced positive CPE culture isolates from surveillance cultures that had the same CPE enzyme as that of the index case patients.
Results
A total of 211 index case patients and 2,689 corresponding contact patients were identified. Of the contact patients, 1,369 (50.9%) including 649 New-Delhi metallo-betalactamase-1 (NDM-1) and 448 Klebsiella pneumoniae carbaepenamse (KPC)-producing CPE exposures were screened, and 44 secondary patients (3.2%; 95% confidence interval 2.3 -4.3%) were positive for NDM-1-producing CPE (16 patients) and KPC-producing (24 patients) CPE. The CPE acquisition rate (5.4%) for KPC-producing CPE exposures was significantly higher than that for NDM-1 exposures (2.7%) (P = 0.01).
Conclusion
The CPE acquisition rate was 3.2% among close contacts sharing a multi-patient room, with about a two-fold higher risk of KPC-producing CPE than NDM-1-producing CPE.
Keyword
Figure
Reference
-
1. Logan LK, Weinstein RA. The epidemiology of carbapenem-resistant Enterobacteriaceae: the impact and evolution of a global menace. J Infect Dis. 2017; 215(suppl_1):S28–S36. PMID: 28375512.
Article2. Peleg AY, Hooper DC. Hospital-acquired infections due to gram-negative bacteria. N Engl J Med. 2010; 362:1804–1813. PMID: 20463340.
Article3. Marston HD, Dixon DM, Knisely JM, Palmore TN, Fauci AS. Antimicrobial resistance. JAMA. 2016; 316:1193–1204. PMID: 27654605.
Article4. Nordmann P, Cuzon G, Naas T. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect Dis. 2009; 9:228–236. PMID: 19324295.5. Lee E, Lee S, Park H, Kim S, Lee H. Analysis of carbapenemase-producing Enterobacteriaceae (CPE) surveillance results for 2017 in Korea: Comparison with the surveillance results of the previous 5 years (2012-2016). PHWR. 2018; 11:1586–1594.6. Cardoso T, Almeida M, Friedman ND, Aragão I, Costa-Pereira A, Sarmento AE, Azevedo L. Classification of healthcare-associated infection: a systematic review 10 years after the first proposal. BMC Med. 2014; 12:40. PMID: 24597462.
Article7. Korean Society for Healthcare-associated Infection Control and Prevention (KOSHIC). Korean national healthcare-associated infections surveillance system manual. 2018.8. Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing: M100. 27th ed. Wayne, PA: CLSI;2017.9. Podschun R, Ullmann U. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev. 1998; 11:589–603. PMID: 9767057.10. Lerner A, Adler A, Abu-Hanna J, Cohen Percia S, Kazma Matalon M, Carmeli Y. Spread of KPC-producing carbapenem-resistant Enterobacteriaceae: the importance of super-spreaders and rectal KPC concentration. Clin Microbiol Infect. 2015; 21:470.e1–470.e7.
Article11. Lim YJ, Park HY, Lee JY, Kwak SH, Kim MN, Sung H, Kim SH, Choi SH. Clearance of carbapenemase-producing Enterobacteriaceae (CPE) carriage: a comparative study of NDM-1 and KPC CPE. Clin Microbiol Infect. 2018; 24:1104.e5–1104.e8.12. Walsh TR, Weeks J, Livermore DM, Toleman MA. Dissemination of NDM-1 positive bacteria in the New Delhi environment and its implications for human health: an environmental point prevalence study. Lancet Infect Dis. 2011; 11:355–362. PMID: 21478057.
Article13. Seara N, Oteo J, Carrillo R, Pérez-Blanco V, Mingorance J, Gómez-Gil R, Herruzo R, Pérez-Vázquez M, Astray J, García-Rodríguez J, Ruiz-Velasco LM, Campos J, de Burgos C, Ruiz-Carrascoso G. Interhospital spread of NDM-7-producing Klebsiella pneumoniae belonging to ST437 in Spain. Int J Antimicrob Agents. 2015; 46:169–173. PMID: 25982912.
Article14. Schwartz-Neiderman A, Braun T, Fallach N, Schwartz D, Carmeli Y, Schechner V. Risk factors for carbapenemase-producing carbapenem-resistant Enterobacteriaceae (CP-CRE) acquisition among contacts of newly diagnosed CP-CRE patients. Infect Control Hosp Epidemiol. 2016; 37:1219–1225. PMID: 27452597.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Carbapenem-resistant Enterobacteriaceae in Korea
- Carbapenem Inactivation Method: Accurate Detection and Easy Interpretation of Carbapenemase Production in Enterobacteriaceae and Pseudomonas spp.
- Epidemiological Characteristics of Carbapenemase Producing Carbapenem-Resistant Enterobacteriaceae Colonization
- Rapid Identification of OXA-48-like, KPC, NDM, and VIM Carbapenemase-Producing Enterobacteriaceae From Culture: Evaluation of the RESIST-4 O.K.N.V. Multiplex Lateral Flow Assay
- Emergence and Spread of OXA-48-Like Carbapenemase-Producing Enterobacteriaceae