J Liver Cancer.
2020 Mar;20(1):72-77. 10.17998/jlc.20.1.72.
Nivolumab for Advanced Hepatocellular Carcinoma with Multiple Lung Metastases after Sorafenib Failure
- Affiliations
-
- 1Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea
- 2Institute of Gastroenterology, Yonsei University College of Medicine, Seoul, Korea
- 3Yonsei Liver Center, Yonsei University Health System, Seoul, Korea
- KMID: 2505845
- DOI: http://doi.org/10.17998/jlc.20.1.72
Abstract
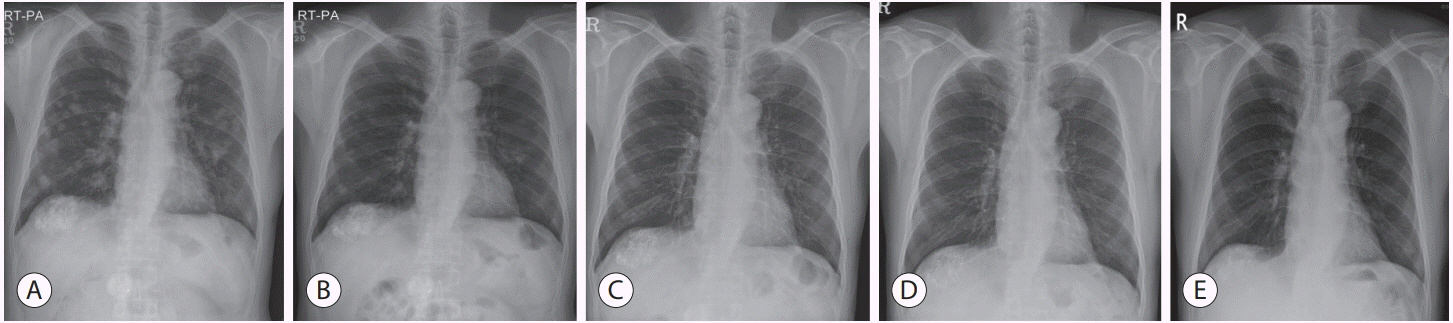
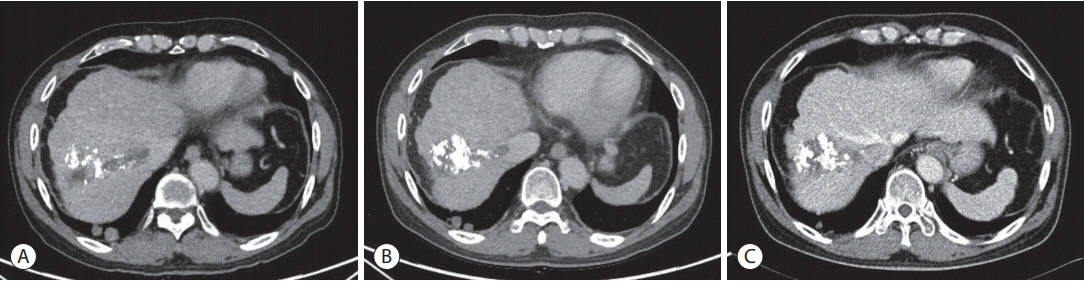
- Over the past decade, standard first-line systemic treatment of advanced hepatocellular carcinoma (HCC) has been based on sorafenib, a multi-kinase inhibitor. Regorafenib, another tyrosine kinase inhibitor, is the only second-line therapy that has been globally approved after progression under sorafenib treatment. Recently, immunotherapeutic agents have emerged as promising treatment options in many different malignancies, including advanced HCC. Nivolumab is the first immunotherapy approved by the Food and Drug Administration for use in HCC patients with advanced-stage second-line after sorafenib failure. In this report, a case of advanced HCC with multiple lung metastases in which a complete response and maintained progression-free status was achieved with nivolumab, following the failure of transarterial chemoembolization and sorafenib is presented. We hope this report may help expand the clinical application of second-line treatment.
Figure
Reference
-
1. Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019; 380:1450–1462.2. Korean Liver Cancer Association; National Cancer Center. 2018 Korean Liver Cancer Association-National Cancer Center Korea Practice Guidelines for the Management of Hepatocellular Carcinoma. Gut Liver. 2019; 13:227–299.3. Lee JS, Cho IR, Lee HW, Jeon MY, Lim TS, Baatarkhuu O, et al. Conditional survival estimates improve over time for patients with hepatocellular carcinoma: An Analysis for Nationwide Korea Cancer Registry Database. Cancer Res Treat. 2019; 51:1347–1356.4. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008; 359:378–390.5. Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009; 10:25–34.6. Cheng AL, Guan Z, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma according to baseline status: subset analyses of the phase III Sorafenib Asia-Pacific trial. Eur J Cancer. 2012; 48:1452–1465.7. Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, doubleblind, placebo-controlled, phase 3 trial. Lancet. 2017; 389:56–66.8. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012; 12:252–264.9. Emens LA, Ascierto PA, Darcy PK, Demaria S, Eggermont AMM, Redmond WL, et al. Cancer immunotherapy: opportunities and challenges in the rapidly evolving clinical landscape. Eur J Cancer. 2017; 81:116–129.10. Whiteside TL, Demaria S, Rodriguez-Ruiz ME, Zarour HM, Melero I. Emerging opportunities and challenges in cancer immunotherapy. Clin Cancer Res. 2016; 22:1845–1855.11. Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017; 390:2461–2471.12. Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015; 373:23–34.13. Harrington KJ, Ferris RL, Blumenschein G Jr, Colevas AD, Fayette J, Licitra L, et al. Nivolumab versus standard, single-agent therapy of investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck (CheckMate 141): health-related quality-of-life results from a randomised, phase 3 trial. Lancet Oncol. 2017; 18:1104–1115.14. El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017; 389:2492–2502.15. Chon YE, Park H, Hyun HK, Ha Y, Kim MN, Kim BK, et al. Development of a new Nomogram including neutrophil-to-lymphocyte ratio to predict survival in patients with hepatocellular carcinoma undergoing transarterial chemoembolization. Cancers (Basel). 2019; 11:E509.16. Cho Y, Han J, Kim W. Recent advances and future directions in immunotherapeutics for hepatocellular carcinoma. J Liver Cancer. 2019; 19:1–11.17. Lee HW, Cho KJ, Park JY. Current status and future direction of immunotherapy in hepatocellular carcinoma: what do the data suggest? Immune Netw. 2020; 20:e11.18. Yau T, Park J, Finn R, Cheng A, Mathurin P, Edeline J, et al. Check-Mate 459: A randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Ann Oncol. 2019; 30:v874–v875.19. Lee HW, Cho KJ, Shin SY, Kim HY, Lee EJ, Kim BK, et al. Serum PD-1 levels change with immunotherapy response but do not predict prognosis in patients with hepatocellular carcinoma. J Liver Cancer. 2019; 19:108–116.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Infiltrative hepatocellular carcinoma with multiple lung metastasis completely cured using nivolumab: a case report
- A Case of Achieving Partial Remission with the Combination of Sorafenib and Nivolumab in a Patient with Hepatocellular Carcinoma Showing Disease Progression after Nivolumab Therapy
- A case of nearly complete response in hepatocellular carcinoma with disseminated lung metastasis by combination therapy of nivolumab and ipilimumab after treatment failure of atezolizumab plus bevacizumab
- Systemic Therapy for Advanced Hepatocellular Carcinoma: Targeted Therapy and Immunotherapy
- Treatment options after sorafenib failure in patients with hepatocellular carcinoma