Korean J Physiol Pharmacol.
2020 Sep;24(5):395-402. 10.4196/kjpp.2020.24.5.395.
Effects of troxerutin on vascular inflammatory mediators andexpression of microRNA-146a/NF-B signaling pathway in aortaof healthy and diabetic rats
- Affiliations
-
- 1Department of Vascular Surgery, Taizhou People’s Hospital, Taizhou, Jiangsu province 225300, China
- KMID: 2505774
- DOI: http://doi.org/10.4196/kjpp.2020.24.5.395
Abstract
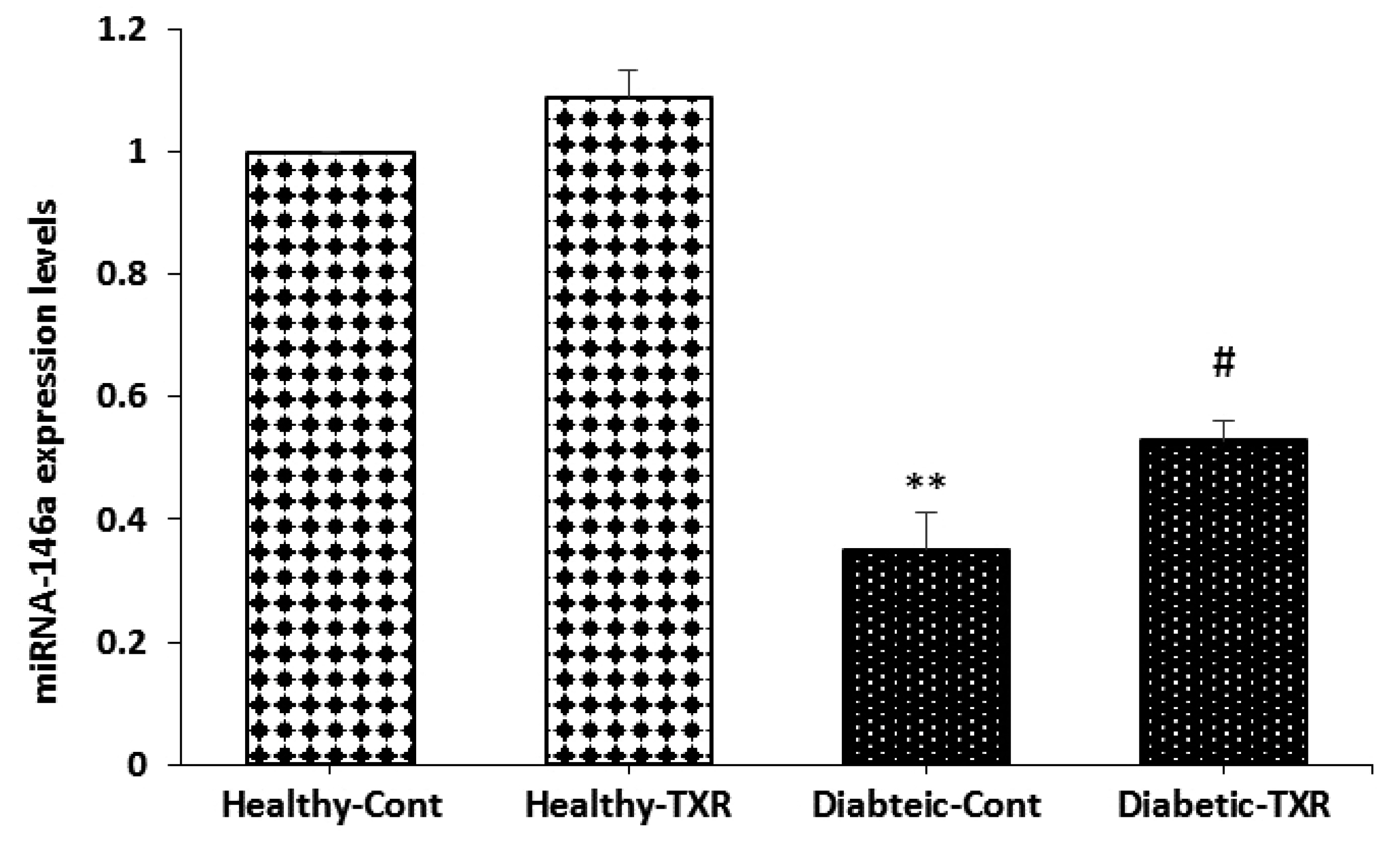
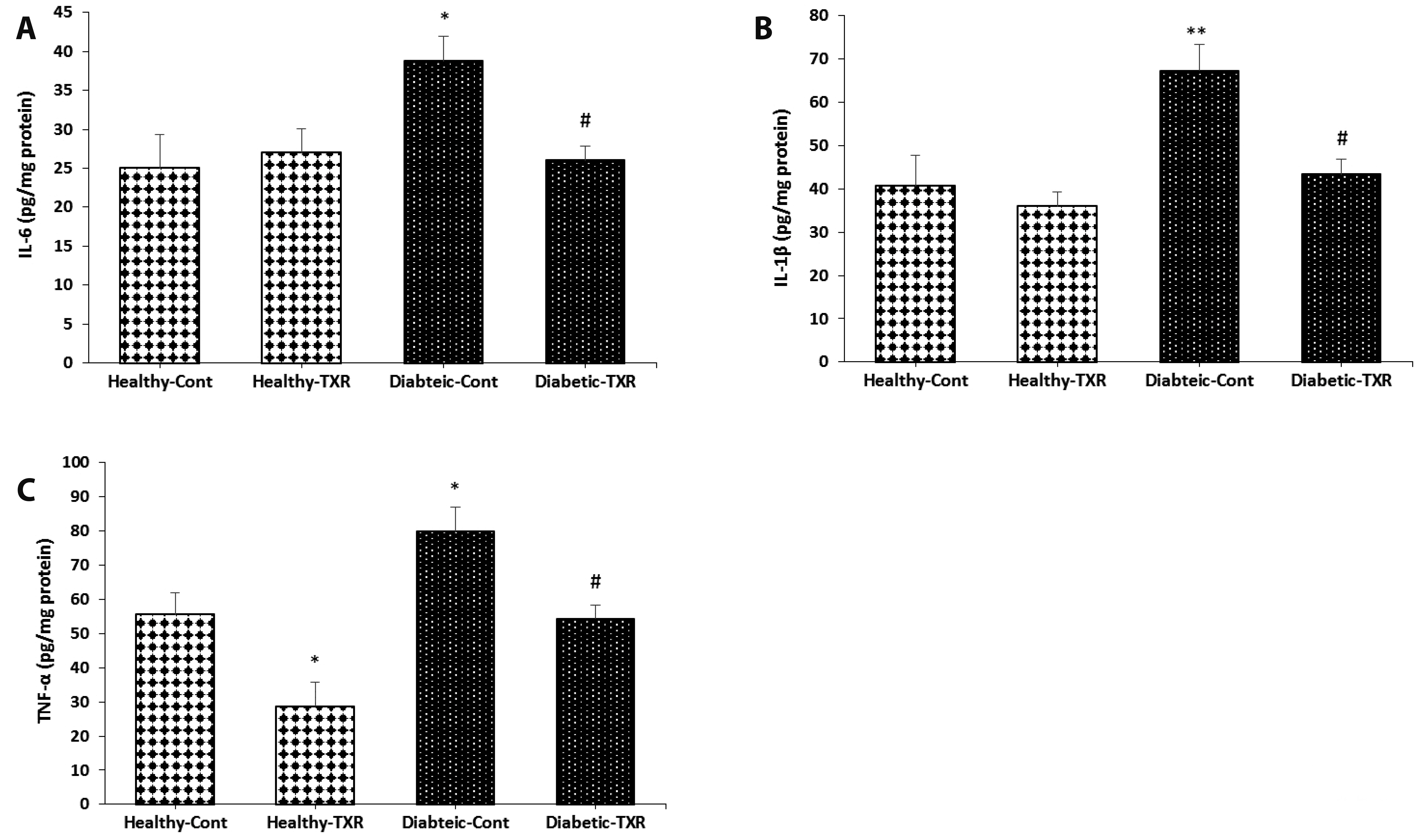
- This study has investigated the effect of a potent bioflavonoid, troxerutin,on diabetes-induced changes in pro-inflammatory mediators and expression ofmicroRNA-146a and nuclear factor-kappa-B (NF-κB) signaling pathway in aortic tissueof type-I diabetic rats. Male Wistar rats were randomly divided into four groups(n = 6/each): healthy, healthy-troxerutin, diabetic, and diabetic-troxerutin. Diabeteswas induced by streptozotocin injection (60 mg/kg; intraperitoneally) and lasted 10weeks. Troxerutin (150 mg/kg/day) was administered orally for last month of experiment.Inflammatory cytokines IL-1, IL-6, and TNF-, as well as intercellular adhesionmolecule-1 (ICAM-1), vascular cell adhesion molecule (VCAM), cyclooxygenase-II(COX-II), and inducible-nitric oxide synthase (iNOS) were measured on aortic samplesby enzyme-linked immunosorbent assay. Gene expressions for transcription factorNF-κB, interleukin-1 receptor-associated kinase-1 (IRAK-1), TNF receptor-associatedfactor-6 (TRAF-6), and microRNA-146a were determined using real-time polymerasechain reaction. Ten-week diabetes significantly increased mRNA levels of IRAK-1,TRAF-6, NF-κB, and protein levels of cytokines IL-1, IL-6, TNF-, adhesion moleculesICAM-1, VCAM, and iNOS, COX-II, and decreased expression of microRNA-146a ascompared with healthy rats (p < 0.05 to p < 0.01). However, one month treatmentof diabetic rats with troxerutin restored glucose and insulin levels, significantly decreasedexpression of inflammatory genes and pro-inflammatory mediators andincreased microRNA level in comparison to diabetic group (p < 0.05 to p < 0.01). Inhealthy rats, troxerutin had significant reducing effect only on NF-κB, TNF- and COXIIlevels (p < 0.05). Beside slight improvement of hyperglycemia, troxerutin preventedthe activation of NF-κB-dependent inflammatory signaling in
Figure
Reference
-
1. Rask-Madsen C, King GL. 2013; Vascular complications of diabetes: mechanisms of injury and protective factors. Cell Metab. 17:20–33. DOI: 10.1016/j.cmet.2012.11.012. PMID: 23312281. PMCID: PMC3546345.
Article2. Nicholls SJ, Tuzcu EM, Kalidindi S, Wolski K, Moon KW, Sipahi I, Schoenhagen P, Nissen SE. 2008; Effect of diabetes on progression of coronary atherosclerosis and arterial remodeling: a pooled analysis of 5 intravascular ultrasound trials. J Am Coll Cardiol. 52:255–262. DOI: 10.1016/j.jacc.2008.03.051. PMID: 18634979.3. Shi Y, Vanhoutte PM. 2017; Macro- and microvascular endothelial dysfunction in diabetes. J Diabetes. 9:434–449. DOI: 10.1111/1753-0407.12521. PMID: 28044409.
Article4. Yuan T, Yang T, Chen H, Fu D, Hu Y, Wang J, Yuan Q, Yu H, Xu W, Xie X. 2019; New insights into oxidative stress and inflammation during diabetes mellitus-accelerated atherosclerosis. Redox Biol. 20:247–260. DOI: 10.1016/j.redox.2018.09.025. PMID: 30384259. PMCID: PMC6205410.
Article5. Kantharidis P, Wang B, Carew RM, Lan HY. 2011; Diabetes complications: the microRNA perspective. Diabetes. 60:1832–1837. DOI: 10.2337/db11-0082. PMID: 21709278. PMCID: PMC3121430.
Article6. Feng B, Chen S, McArthur K, Wu Y, Sen S, Ding Q, Feldman RD, Chakrabarti S. 2011; miR-146a-mediated extracellular matrix protein production in chronic diabetes complications. Diabetes. 60:2975–2984. DOI: 10.2337/db11-0478. PMID: 21885871. PMCID: PMC3198068.
Article7. Bhatt K, Lanting LL, Jia Y, Yadav S, Reddy MA, Magilnick N, Boldin M, Natarajan R. 2016; Anti-inflammatory role of microRNA-146a in the pathogenesis of diabetic nephropathy. J Am Soc Nephrol. 27:2277–2288. DOI: 10.1681/ASN.2015010111. PMID: 26647423. PMCID: PMC4978034.
Article8. Feng Y, Chen L, Luo Q, Wu M, Chen Y, Shi X. 2018; Involvement of microRNA-146a in diabetic peripheral neuropathy through the regulation of inflammation. Drug Des Devel Ther. 12:171–177. DOI: 10.2147/DDDT.S157109. PMID: 29398906. PMCID: PMC5775734.
Article9. Lu J, Wu DM, Zheng YL, Hu B, Cheng W, Zhang ZF, Li MQ. 2013; Troxerutin counteracts domoic acid-induced memory deficits in mice by inhibiting CCAAT/enhancer binding protein β-mediated inflammatory response and oxidative stress. J Immunol. 190:3466–3479. DOI: 10.4049/jimmunol.1202862. PMID: 23420885.
Article10. Geetha R, Radika MK, Priyadarshini E, Bhavani K, Anuradha CV. 2015; Troxerutin reverses fibrotic changes in the myocardium of high-fat high-fructose diet-fed mice. Mol Cell Biochem. 407:263–279. DOI: 10.1007/s11010-015-2474-3. PMID: 26077659.
Article11. Zhang S, Li H, Zhang L, Li J, Wang R, Wang M. 2017; Effects of troxerutin on cognitive deficits and glutamate cysteine ligase subunits in the hippocampus of streptozotocin-induced type 1 diabetes mellitus rats. Brain Res. 1657:355–360. DOI: 10.1016/j.brainres.2016.12.009. PMID: 27998794.
Article12. Shu L, Zhang W, Huang G, Huang C, Zhu X, Su G, Xu J. 2019; Troxerutin attenuates myocardial cell apoptosis following myocardial ischemia-reperfusion injury through inhibition of miR-146a-5p expression. J Cell Physiol. 234:9274–9282. DOI: 10.1002/jcp.27607. PMID: 30417352.
Article13. Zabihi NA, Mousavi SM, Mahmoudabady M, Soukhtanloo M, Sohrabi F, Niazmand S. 2018; Teucrium polium L. improves blood glucose and lipids and ameliorates oxidative stress in heart and aorta of diabetic rats. Int J Prev Med. 9:110. DOI: 10.4103/ijpvm.IJPVM_189_17. PMID: 30687461. PMCID: PMC6326021.
Article14. Najafi M, Farajnia S, Mohammadi M, Badalzadeh R, Ahmadi Asl N, Baradaran B, Amani M. 2014; Inhibition of mitochondrial permeability transition pore restores the cardioprotection by postconditioning in diabetic hearts. J Diabetes Metab Disord. 13:106. DOI: 10.1186/s40200-014-0106-1. PMID: 25436201. PMCID: PMC4247617.
Article15. Sampath S, Karundevi B. 2014; Effect of troxerutin on insulin signaling molecules in the gastrocnemius muscle of high fat and sucrose-induced type-2 diabetic adult male rat. Mol Cell Biochem. 395:11–27. DOI: 10.1007/s11010-014-2107-2. PMID: 24880482.
Article16. Schalkwijk CG, Stehouwer CD. 2005; Vascular complications in diabetes mellitus: the role of endothelial dysfunction. Clin Sci (Lond). 109:143–159. DOI: 10.1042/CS20050025. PMID: 16033329.
Article17. Lam TY, Seto SW, Lau YM, Au LS, Kwan YW, Ngai SM, Tsui KW. 2006; Impairment of the vascular relaxation and differential expression of caveolin-1 of the aorta of diabetic +db/+db mice. Eur J Pharmacol. 546:134–141. DOI: 10.1016/j.ejphar.2006.07.003. PMID: 16904102.
Article18. Mohammad A, Ali N, Reza B, Ali K. 2010; Effect of ascorbic acid supplementation on nitric oxide metabolites and systolic blood pressure in rats exposed to lead. Indian J Pharmacol. 42:78–81. DOI: 10.4103/0253-7613.64501. PMID: 20711370. PMCID: PMC2907019.
Article19. Das Evcimen N, King GL. 2007; The role of protein kinase C activation and the vascular complications of diabetes. Pharmacol Res. 55:498–510. DOI: 10.1016/j.phrs.2007.04.016. PMID: 17574431.20. Bullon P, Newman HN, Battino M. 2014; Obesity, diabetes mellitus, atherosclerosis and chronic periodontitis: a shared pathology via oxidative stress and mitochondrial dysfunction? Periodontol 2000. 64:139–153. DOI: 10.1111/j.1600-0757.2012.00455.x. PMID: 24320961.
Article21. Kim F, Pham M, Maloney E, Rizzo NO, Morton GJ, Wisse BE, Kirk EA, Chait A, Schwartz MW. 2008; Vascular inflammation, insulin resistance, and reduced nitric oxide production precede the onset of peripheral insulin resistance. Arterioscler Thromb Vasc Biol. 28:1982–1988. DOI: 10.1161/ATVBAHA.108.169722. PMID: 18772497. PMCID: PMC2577575.
Article22. Basta G, Schmidt AM, De Caterina R. 2004; Advanced glycation end products and vascular inflammation: implications for accelerated atherosclerosis in diabetes. Cardiovasc Res. 63:582–592. DOI: 10.1016/j.cardiores.2004.05.001. PMID: 15306213.
Article23. Dasu MR, Devaraj S, Zhao L, Hwang DH, Jialal I. 2008; High glucose induces toll-like receptor expression in human monocytes: mechanism of activation. Diabetes. 57:3090–3098. DOI: 10.2337/db08-0564. PMID: 18650365. PMCID: PMC2570406.
Article24. Patel S, Santani D. 2009; Role of NF-kappa B in the pathogenesis of diabetes and its associated complications. Pharmacol Rep. 61:595–603. DOI: 10.1016/S1734-1140(09)70111-2.25. Yu Y, Zheng G. 2017; Troxerutin protects against diabetic cardiomyopathy through NF-κB/AKT/IRS1 in a rat model of type 2 diabetes. Mol Med Rep. 15:3473–3478. DOI: 10.3892/mmr.2017.6456. PMID: 28440404. PMCID: PMC5436284.
Article26. Zhang H, Liu J, Qu D, Wang L, Luo JY, Lau CW, Liu P, Gao Z, Tipoe GL, Lee HK, Ng CF, Ma RC, Yao X, Huang Y. 2016; Inhibition of miR-200c restores endothelial function in diabetic mice through suppression of COX-2. Diabetes. 65:1196–1207. DOI: 10.2337/db15-1067. PMID: 26822089.
Article27. Yang M, Ye L, Wang B, Gao J, Liu R, Hong J, Wang W, Gu W, Ning G. 2015; Decreased miR-146 expression in peripheral blood mononuclear cells is correlated with ongoing islet autoimmunity in type 1 diabetes patients. J Diabetes. 7:158–165. DOI: 10.1111/1753-0407.12163. PMID: 24796653.28. García-Díaz DF, Pizarro C, Camacho-Guillén P, Codner E, Soto N, Pérez-Bravo F. 2018; Expression of miR-155, miR-146a, and miR-326 in T1D patients from Chile: relationship with autoimmunity and inflammatory markers. Arch Endocrinol Metab. 62:34–40. DOI: 10.20945/2359-3997000000006. PMID: 29694627.
Article29. Feng B, Chen S, Gordon AD, Chakrabarti S. 2017; miR-146a mediates inflammatory changes and fibrosis in the heart in diabetes. J Mol Cell Cardiol. 105:70–76. DOI: 10.1016/j.yjmcc.2017.03.002. PMID: 28279663.
Article30. Mann M, Mehta A, Zhao JL, Lee K, Marinov GK, Garcia-Flores Y, Lu LF, Rudensky AY, Baltimore D. 2017; An NF-κB-microRNA regulatory network tunes macrophage inflammatory responses. Nat Commun. 8:851. DOI: 10.1038/s41467-017-00972-z. PMID: 29021573. PMCID: PMC5636846.
Article31. Ye EA, Steinle JJ. 2016; miR-146a attenuates inflammatory pathways mediated by TLR4/NF-κB and TNFα to protect primary human retinal microvascular endothelial cells grown in high glucose. Mediators Inflamm. 2016:3958453. DOI: 10.1155/2016/3958453. PMID: 26997759. PMCID: PMC4779539.32. Huang Y, Liu Y, Li L, Su B, Yang L, Fan W, Yin Q, Chen L, Cui T, Zhang J, Lu Y, Cheng J, Fu P, Liu F. 2014; Involvement of inflammation-related miR-155 and miR-146a in diabetic nephropathy: implications for glomerular endothelial injury. BMC Nephrol. 15:142. DOI: 10.1186/1471-2369-15-142. PMID: 25182190.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Atorvastatin Pretreatment Ameliorates Mesenchymal Stem Cell Migration through miR-146a/CXCR4 Signaling
- MIR210HG Aggravates Sepsis-Induced Inflammatory Response of Proximal Tubular Epithelial Cell via the NF-κB Signaling Pathway
- L-Methionine inhibits 4-hydroxy-2-nonenal accumulation and suppresses inflammation in growing rats
- Bacterial PAMPs and Allergens Trigger Increase in [Ca2+]i-induced Cytokine Expression in Human PDL Fibroblasts
- Activation of Protein Tyrosine Kinase Pathway in Diabetic Rats