J Korean Med Sci.
2020 Aug;35(32):e253. 10.3346/jkms.2020.35.e253.
Pulmonary Surfactant Replacement Therapy for Respiratory Distress Syndrome in Neonates: a Nationwide Epidemiological Study in Korea
- Affiliations
-
- 1Department of Pediatrics, Yonsei University College of Medicine, Seoul, Korea
- KMID: 2505577
- DOI: http://doi.org/10.3346/jkms.2020.35.e253
Abstract
- Background
Pulmonary surfactant (PS) replacement therapy, as a safe and effective treatment for respiratory distress syndrome (RDS) may have further increased with the extended insurance coverage since 2011 in Korea. Thus, this study aimed to investigate the epidemiologic data of PS replacement therapy for RDS in Korea and to analyze the complications associated with RDS.
Methods
We included 19,442 infants who were treated with PS and diagnosed with RDS (International Classification of Diseases-10 codes: P22.0) between 2014 and 2018 from the Health Insurance Review and Assessment database. Birth certificate data from Statistics Korea were used to estimate the incidence of RDS.
Results
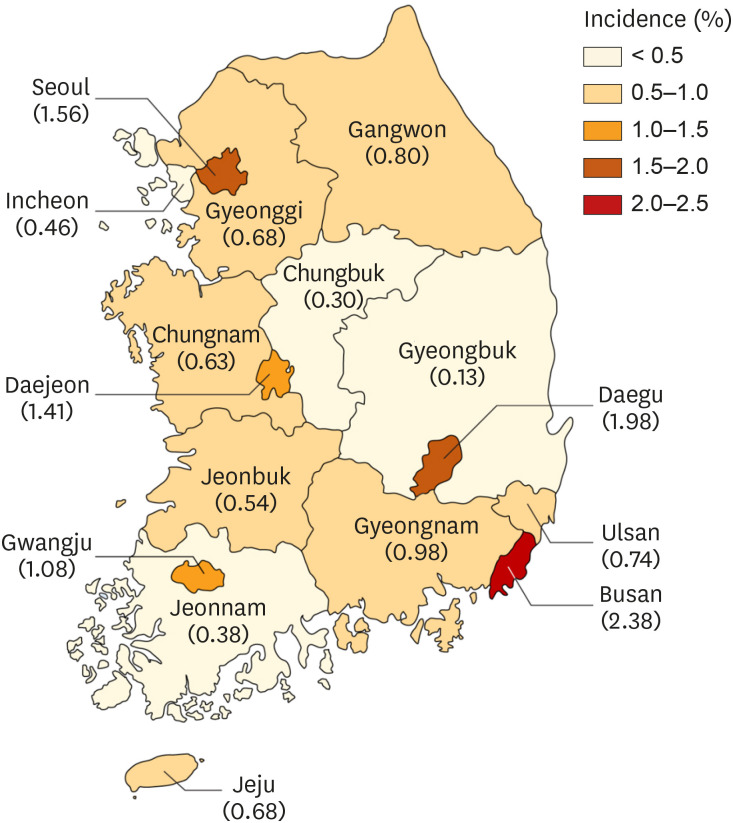
The average incidence of RDS within the study period was 0.99% among live births. Repeated doses of PS were administered to 1,688 infants (8.7%), ranging from 2 doses in 929 infants (4.8%) to 9 doses in 1 infant (0.01%). The incidence of RDS in term infants markedly increased over 5 years from 0.2% to 0.34%. The incidence was similarly increased among the preterm infants. The RDS mortality rate was 6.3% and showed a decreasing trend according to year. The mortality rate was significantly higher in the lower gestational age group. A decreasing trend was observed in the incidence of the complications, such as patent ductus arteriosus, intraventricular hemorrhage, and bronchopulmonary dysplasia, except for pneumothorax in term infants. The complications were also higher in the lower gestational age group and the lower birth weight group. However, pneumothorax was the most frequent complication in the term infant group and in infants with birth weight ≥ 2,500 g.
Conclusion
Advancements in neonatal care and extended insurance coverage have increased the use of PS replacement therapy for RDS. This, in turn, decreased neonatal mortality and the incidence of the associated complications. The appropriate therapeutic strategy for RDS should be decided according to the gestational age and lung pathology.
Keyword
Figure
Reference
-
1. Engle WA. American Academy of Pediatrics Committee on Fetus and Newborn. Surfactant-replacement therapy for respiratory distress in the preterm and term neonate. Pediatrics. 2008; 121(2):419–432. PMID: 18245434.
Article2. Polin RA, Carlo WA. Committee on Fetus and Newborn. American Academy of Pediatrics. Surfactant replacement therapy for preterm and term neonates with respiratory distress. Pediatrics. 2014; 133(1):156–163. PMID: 24379227.
Article3. Soll R, Ozek E. Multiple versus single doses of exogenous surfactant for the prevention or treatment of neonatal respiratory distress syndrome. Cochrane Database Syst Rev. 2009; (1):CD000141. PMID: 19160177.
Article4. Moya F, Sinha S, D'Agostino RB Sr. Surfactant-replacement therapy for respiratory distress syndrome in the preterm and term neonate: congratulations and corrections. Pediatrics. 2008; 121(6):1290–1291. PMID: 18519504.
Article5. Bae CW, Kim CY, Chung SH, Choi YS. History of pulmonary surfactant replacement therapy for neonatal respiratory distress syndrome in Korea. J Korean Med Sci. 2019; 34(25):e175. PMID: 31243934.
Article6. Bae CW. The history of neonatology in Korea. J Korean Med Assoc. 2016; 59(7):490–497.
Article7. Shim JW, Jin HS, Bae CW. Changes in survival rate for very-low-birth-weight infants in Korea: comparison with other countries. J Korean Med Sci. 2015; 30(Suppl 1):S25–S34. PMID: 26566354.
Article8. Hahn WH, Chang JY, Chang YS, Shim KS, Bae CW. Recent trends in neonatal mortality in very low birth weight Korean infants: in comparison with Japan and the USA. J Korean Med Sci. 2011; 26(4):467–473. PMID: 21468252.
Article9. Chung SH, Bae CW. Improvement in the survival rates of very low birth weight infants after the establishment of the Korean Neonatal Network: comparison between the 2000s and 2010s. J Korean Med Sci. 2017; 32(8):1228–1234. PMID: 28665056.
Article10. Aldana-Aguirre JC, Pinto M, Featherstone RM, Kumar M. Less invasive surfactant administration versus intubation for surfactant delivery in preterm infants with respiratory distress syndrome: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2017; 102(1):F17–F23. PMID: 27852668.
Article11. Bae CW, Hahn WH, Chang JY, Kim SM. Surfactant replacement therapy for RDS: a collaborative study of 72 multi-center trials in Korea (2010) and a review of Korean experiences over 20 years. J Korean Soc Neonatol. 2011; 18(2):409–411.
Article12. Bae CW, Kim YM. Surfactant therapy for neonatal respiratory distress syndrome: experience in Korea over 15 years. Korean J Pediatr. 2004; 47(9):940–948.13. Bae CW, Hahn WH. Surfactant therapy for neonatal respiratory distress syndrome: a review of Korean experiences over 17 years. J Korean Med Sci. 2009; 24(6):1110–1118. PMID: 19949668.
Article14. Kim L, Kim JA, Kim S. A guide for the utilization of Health Insurance Review and Assessment Service national patient samples. Epidemiol Health. 2014; 36:e2014008. PMID: 25078381.
Article15. Statistics Korea. Updated 2019. Accessed March 31, 2020. http://kosis.kr/eng/statisticsList/statisticsListIndex.do?menuId=M_01_01&vwcd=MT_ETITLE&parmTabId=M_01_01#SelectStatsBoxDiv.16. Bae CW, Kwon YD, Ko SJ, Kim KS, Kim HM, Park WS, et al. Surfactant replacement therapy in neonates with respiratory distress syndrome: a collective evaluation of trials from 16 hospitals. J Korean Pediatr Soc. 1993; 36(2):244–265.17. Bae CW. Surfactant replacement therapy in RDS: a collaborative study of multi-center trials in Korea. J Korean Soc Neonatol. 1997; 4(1):124–135.18. Lim HS, Kim H, Jin JY, Shin YL, Park JO, Kim CH, et al. Characteristics of pneumothorax in a neonatal intensive care unit. J Korean Soc Neonatol. 2011; 18(2):257–264.
Article19. Park SW, Yun BH, Kim KA, Ko SY, Lee YK, Shin SM. A clinical study about symptomatic spontaneous pneumothorax. Korean J Perinatol. 2006; 17(3):304–309.20. Stoelhorst GM, Rijken M, Martens SE, Brand R, den Ouden AL, Wit JM, et al. Changes in neonatology: comparison of two cohorts of very preterm infants (gestational age <32 weeks): the project on preterm and small for gestational age infants 1983 and the Leiden follow-up project on prematurity 1996–1997. Pediatrics. 2005; 115(2):396–405. PMID: 15689337.21. Ventolini G, Neiger R, Mathews L, Adragna N, Belcastro M. Incidence of respiratory disorders in neonates born between 34 and 36 weeks of gestation following exposure to antenatal corticosteroids between 24 and 34 weeks of gestation. Am J Perinatol. 2008; 25(2):79–83. PMID: 18188800.
Article22. The Executive Committee of Korean Neonatal Network. 2018 Korean Neonatal Network Annual Report. Cheongju: Korean Centers for Disease Control and Prevention;2019.23. The Executive Committee of Korean Neonatal Network. 2014 Korean Neonatal Network Annual Report. Cheongju: Korean Centers for Disease Control and Prevention;2015.24. Koivisto M, Marttila R, Kurkinen-Räty M, Saarela T, Pokela ML, Jouppila P, et al. Changing incidence and outcome of infants with respiratory distress syndrome in the 1990s: a population-based survey. Acta Paediatr. 2004; 93(2):177–184. PMID: 15046270.
Article25. Hibbard J, Wilkins I, Sun L, Gregory K, Haberman S, Hoffman M, et al. Respiratory morbidity in late preterm births. JAMA. 2010; 304(4):419–425. PMID: 20664042.
Article26. Thygesen SK, Olsen M, Østergaard JR, Sørensen HT. Respiratory distress syndrome in moderately late and late preterm infants and risk of cerebral palsy: a population-based cohort study. BMJ open. 2016; 6(10):e011643.
Article27. Mathews TJ, MacDorman MF. Infant mortality statistics from the 2005 period linked birth/infant death data set. Natl Vital Stat Rep. 2008; 57(2):1–32.28. Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001; 163(6):1376–1383. PMID: 11371404.
Article29. Condò V, Cipriani S, Colnaghi M, Bellù R, Zanini R, Bulfoni C, et al. Neonatal respiratory distress syndrome: are risk factors the same in preterm and term infants? J Matern Fetal Neonatal Med. 2017; 30(11):1267–1272. PMID: 27399933.
Article30. Tsakalidis C, Giougki E, Karagianni P, Dokos C, Rallis D, Nikolaidis N. Is there a necessity for multiple doses of surfactant for respiratory distress syndrome of premature infants? Turk J Pediatr. 2012; 54(4):368–375. PMID: 23692717.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Sequential Changes of Chest Radiographic Finding after Exogenous Surfactant Replacement Therapy in Neonates with RDS
- A case of acute respiratory distress syndrome due to maternal blood aspiration
- Surfactant replacement therapy in neonatal respiratory distress syndrome
- The use of artificial pulmonary surfactant in neonatal respiratory distress
- Surfactant replacement therapy in adult respiratory distress syndrome