J Korean Assoc Oral Maxillofac Surg.
2020 Aug;46(4):240-249. 10.5125/jkaoms.2020.46.4.240.
Development of a standardized mucositis and osteoradionecrosis animal model using external radiation
- Affiliations
-
- 1Department of Oral and Maxillofacial Surgery, Dental Research Institute, School of Dentistry, Seoul National University, Seoul, Korea
- 2Department of Pharmacology & Dental Therapeutics, Dental Research Institute, School of Dentistry, Seoul National University, Seoul, Korea
- 3Laboratory Animal Center, Korea Institute of Radiological and Medical Sciences, Seoul, Korea
- 4Department of Oral Pathology, College of Dentistry, Gangneung-Wonju National University, Gangneung, Korea
- KMID: 2505440
- DOI: http://doi.org/10.5125/jkaoms.2020.46.4.240
Abstract
Objectives
Although the side effects of radiation therapy vary from mucositis to osteomyelitis depending on the dose of radiation therapy, to date, an experimental animal model has not yet been proposed. The aim of this study was to develop an animal model for assessing complications of irradiated bone, especially to quantify the dose of radiation needed to develop a rat model.
Materials and Methods
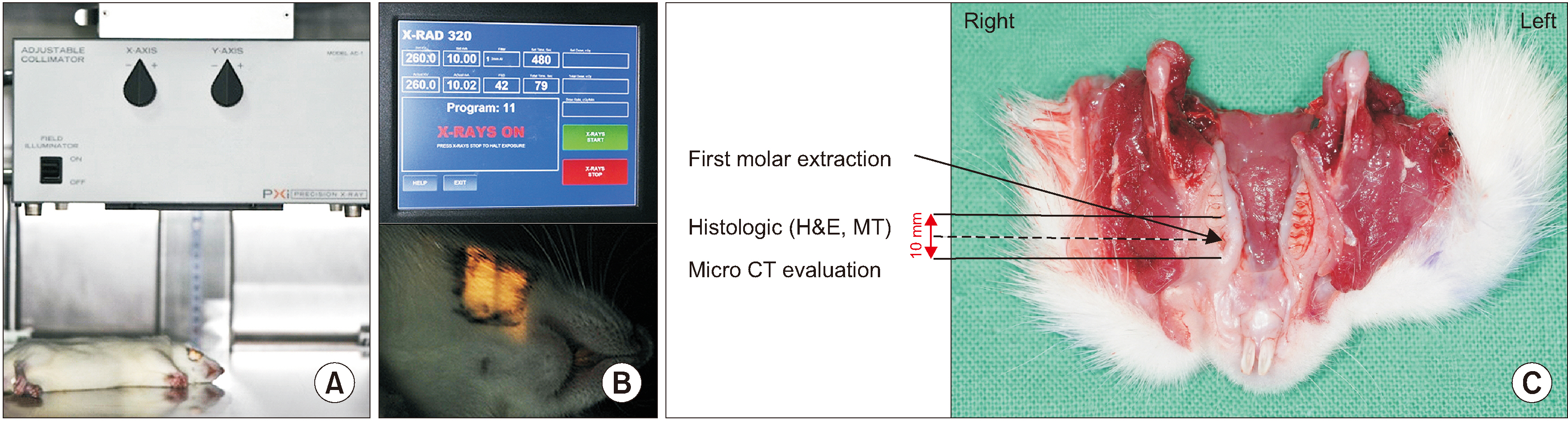
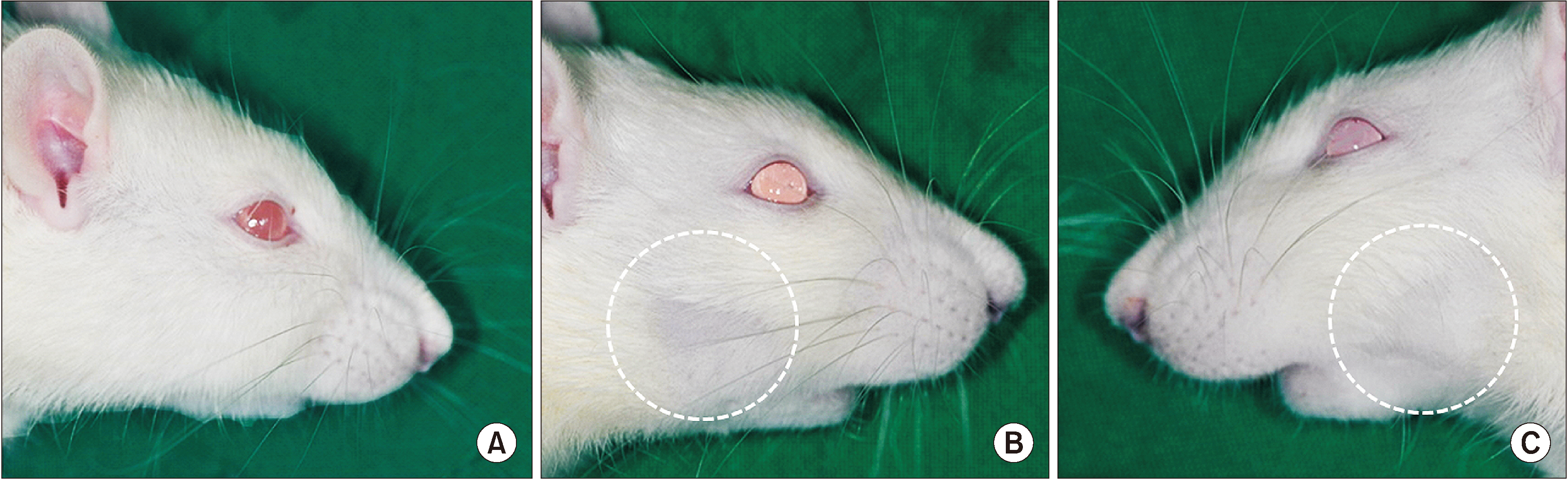
Sixteen Sprague-Dawley rats aged seven weeks with a mean weight of 267.59 g were used. Atraumatic extraction of a right mandibular first molar was performed. At one week after the extraction, the rats were randomized into four groups and received a single dose of external radiation administered to the right lower jaw at a level of 14, 16, 18, or 20 Gy, respectively. Clinical alopecia with body weight changes were compared and bony volumetric analysis with micro-computed tomography (CT), histologic analysis with H&E were performed.
Results
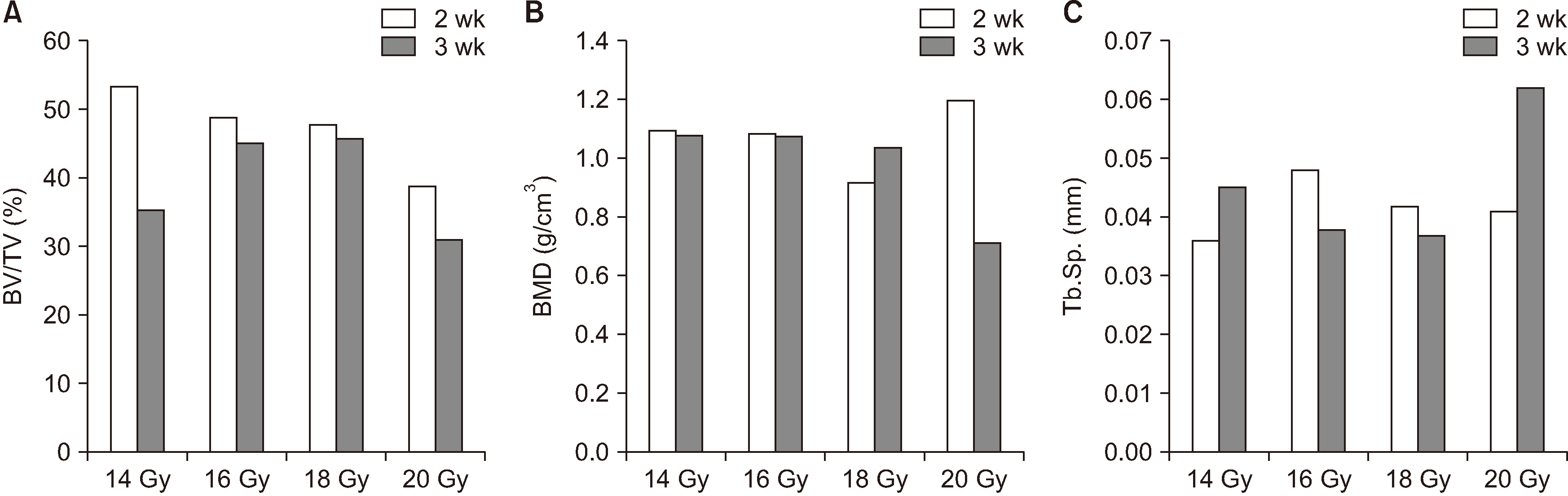
The progression of the skin alopecia was different depending on the irradiation dose. Micro-CT parameters including bone volume, bone volume/tissue volume, bone mineral density, and trabecular spaces, showed no significant differences. The progression of osteoradionecrosis (ORN) along with that of inflammation, fibrosis, and bone resorption, was found with increased osteoclast or fibrosis in the radiated group. As the radiation dose increases, osteoclast numbers begin to decrease and osteoclast tends to increase. Osteoclasts respond more sensitively to the radiation dose, and osteoblasts are degraded at doses above 18 Gy.
Conclusion
A standardized animal model clinically comparable to ORN of the jaw is a valuable tool that can be used to examine the pathophysiology of the disease and trial any potential treatment modalities. We present a methodology for the use of an experimental rat model that incorporates a guideline regarding radiation dose.
Keyword
Figure
Reference
-
References
1. Kim SM, Woo KM, Myoung H, Lee JH, Lee SK. 2015; Tissue engineering treatment in osteomyelitis of the jaws. Tissue Eng Regen Med. 12:11–26. https://doi.org/10.1007/s13770-013-0414-4 . DOI: 10.1007/s13770-013-0414-4.
Article2. Støre G, Boysen M. 2000; Mandibular osteoradionecrosis: clinical behaviour and diagnostic aspects. Clin Otolaryngol Allied Sci. 25:378–84. https://doi.org/10.1046/j.1365-2273.2000.00367.x . DOI: 10.1046/j.1365-2273.2000.00367.x. PMID: 11012651.
Article3. Costa DA, Costa TP, Netto EC, Joaquim N, Ventura I, Pratas AC, et al. 2016; New perspectives on the conservative management of osteoradionecrosis of the mandible: a literature review. Head Neck. 38:1708–16. https://doi.org/10.1002/hed.24495 . DOI: 10.1002/hed.24495. PMID: 27240248.
Article4. Marx RE. 1983; Osteoradionecrosis: a new concept of its pathophysiology. J Oral Maxillofac Surg. 41:283–8. https://doi.org/10.1016/0278-2391(83)90294-x . DOI: 10.1016/0278-2391(83)90294-X. PMID: 6572704.
Article5. Delanian S, Lefaix JL. 2004; The radiation-induced fibroatrophic process: therapeutic perspective via the antioxidant pathway. Radiother Oncol. 73:119–31. https://doi.org/10.1016/j.radonc.2004.08.021 . DOI: 10.1016/j.radonc.2004.08.021. PMID: 15542158.
Article6. Monson LA, Jing XL, Donneys A, Farberg AS, Buchman SR. 2013; Dose-response effect of human equivalent radiation in the mandible. J Craniofac Surg. 24:1593–8. https://doi.org/10.1097/SCS.0b013e31826cfeea . DOI: 10.1097/SCS.0b013e31826cfeea. PMID: 24036733. PMCID: PMC5111555.
Article7. Cohen M, Nishimura I, Tamplen M, Hokugo A, Beumer J, Steinberg ML, et al. 2011; Animal model of radiogenic bone damage to study mandibular osteoradionecrosis. Am J Otolaryngol. 32:291–300. https://doi.org/10.1016/j.amjoto.2010.06.001 . DOI: 10.1016/j.amjoto.2010.06.001. PMID: 20719407.
Article8. Vissink A, Burlage FR, Spijkervet FK, Jansma J, Coppes RP. 2003; Prevention and treatment of the consequences of head and neck radiotherapy. Crit Rev Oral Biol Med. 14:213–25. https://doi.org/10.1177/154411130301400306 . DOI: 10.1177/154411130301400306. PMID: 12799324.
Article9. Zhang WB, Zheng LW, Chua D, Cheung LK. 2010; Bone regeneration after radiotherapy in an animal model. J Oral Maxillofac Surg. 68:2802–9. https://doi.org/10.1016/j.joms.2010.04.024 . DOI: 10.1016/j.joms.2010.04.024. PMID: 20727641.
Article10. He J, Qiu W, Zhang Z, Wang Z, Zhang X, He Y. 2011; Effects of irradiation on growth and differentiation-related gene expression in osteoblasts. J Craniofac Surg. 22:1635–40. https://doi.org/10.1097/SCS.0b013e31822e5f66 . DOI: 10.1097/SCS.0b013e31822e5f66. PMID: 21959403.
Article11. Springer IN, Niehoff P, Açil Y, Marget M, Lange A, Warnke PH, et al. 2008; BMP-2 and bFGF in an irradiated bone model. J Craniomaxillofac Surg. 36:210–7. https://doi.org/10.1016/j.jcms.2007.09.001 . DOI: 10.1016/j.jcms.2007.09.001. PMID: 17945502.
Article12. Grimm G. 1969; [Animal experimental studies on the radiation injuries in the mandibles of full grown rabbits. I. Morphological and histochemical findings]. Dtsch Zahn Mund Kieferheilkd Zentralbl Gesamte. 53:307–35. German.13. Grimm G. 1970; [Animal experimental studies on the pathogenesis of radiogenic bone injuries in the mandibles of adult rabbits. II. Histometric data]. Dtsch Zahn Mund Kieferheilkd Zentralbl Gesamte. 54:352–62. German.14. Hamilton SA, Pecaut MJ, Gridley DS, Travis ND, Bandstra ER, Willey JS, et al. 2006; A murine model for bone loss from therapeutic and space-relevant sources of radiation. J Appl Physiol (1985). 101:789–93. https://doi.org/10.1152/japplphysiol.01078.2005 . DOI: 10.1152/japplphysiol.01078.2005. PMID: 16741258.
Article15. Würzler KK, DeWeese TL, Sebald W, Reddi AH. 1998; Radiation-induced impairment of bone healing can be overcome by recombinant human bone morphogenetic protein-2. J Craniofac Surg. 9:131–7. https://doi.org/10.1097/00001665-199803000-00009 . DOI: 10.1097/00001665-199803000-00009. PMID: 9586541.
Article16. Arnold M, Stas P, Kummermehr J, Schultz-Hector S, Trott KR. 1998; Radiation-induced impairment of bone healing in the rat femur: effects of radiation dose, sequence and interval between surgery and irradiation. Radiother Oncol. 48:259–65. https://doi.org/10.1016/s0167-8140(98)00039-5 . DOI: 10.1016/S0167-8140(98)00039-5.
Article17. Ryu SH, Moon SY, Choi EK, Kim JH, Ahn SD, Song SY, et al. 2008; Establishment of a single dose radiation model of oral mucositis in mice. J Korean Soc Ther Radiol Oncol. 26:257–62. https://doi.org/10.3857/jkstro.2008.26.4.257 . DOI: 10.3857/jkstro.2008.26.4.257.
Article18. Kurihashi T, Iwata H, Nasu M, Yosue T. 2002; Experimental study on wound healing of alveolar bone sockets in the rat maxilla after X-ray irradiation. Odontology. 90:35–42. https://doi.org/10.1007/s102660200005 . DOI: 10.1007/s102660200005. PMID: 12955563.
Article19. Niehoff P, Springer IN, Açil Y, Lange A, Marget M, Roldán JC, et al. 2008; HDR brachytherapy irradiation of the jaw - as a new experimental model of radiogenic bone damage. J Craniomaxillofac Surg. 36:203–9. https://doi.org/10.1016/j.jcms.2008.01.003 . DOI: 10.1016/j.jcms.2008.01.003. PMID: 18436449.
Article20. Tamplen M, Trapp K, Nishimura I, Armin B, Steinberg M, Beumer J, et al. 2011; Standardized analysis of mandibular osteoradionecrosis in a rat model. Otolaryngol Head Neck Surg. 145:404–10. https://doi.org/10.1177/0194599811400576 . DOI: 10.1177/0194599811400576. PMID: 21493310.
Article21. Nanashima N, Ito K, Ishikawa T, Nakano M, Nakamura T. 2012; Damage of hair follicle stem cells and alteration of keratin expression in external radiation-induced acute alopecia. Int J Mol Med. 30:579–84. https://doi.org/10.3892/ijmm.2012.1018 . DOI: 10.3892/ijmm.2012.1018. PMID: 22692500.
Article22. Tchanque-Fossuo CN, Monson LA, Farberg AS, Donneys A, Zehtabzadeh AJ, Razdolsky ER, et al. 2011; Dose-response effect of human equivalent radiation in the murine mandible: part I. A histomorphometric assessment. Plast Reconstr Surg. 128:114–21. https://doi.org/10.1097/PRS.0b013e31821741d4 . DOI: 10.1097/PRS.0b013e31821741d4. PMID: 21701328. PMCID: PMC3132535.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- SURGICAL MANAGEMENT OF OSTEORADIONECROSIS OF THE MANDIBLE
- SERIAL OSTEORADIONECROSIS ON BOTH SIDES OF MANDIBLE: A CASE REPORT
- Osteoradionecrosis of the Anterior Thoracic Wall after Radiation Therapy for Breast Cancer
- Osteoradionecrosis of the jaws
- Establishment of a Single Dose Radiation Model of Oral Mucositis in Mice