Clin Exp Otorhinolaryngol.
2020 Aug;13(3):241-248. 10.21053/ceo.2019.01081.
Noise-Induced Change of Cortical Temporal Processing in Cochlear Implant Users
- Affiliations
-
- 1Laboratory of Brain and Cognitive Sciences for Convergence Medicine, Anyang, Korea
- 2Department of Otorhinolaryngology-Head and Neck Surgery, Hallym University College of Medicine, Chuncheon, Korea
- KMID: 2504918
- DOI: http://doi.org/10.21053/ceo.2019.01081
Abstract
Objectives
. Cochlear implant (CI) users typically report impaired ability to understand speech in noise. Speech understanding in CI users decreases with noise due to reduced temporal processing ability, and speech perceptual errors involve stop consonants distinguished by voice onset time (VOT). The current study examined the effects of noise on various speech perception tests while at the same time used cortical auditory evoked potentials (CAEPs) to quantify the change of neural processing of speech sounds caused by noise. We hypothesized that the noise effects on VOT processing can be reflected in N1/P2 measures, the neural changes relate to behavioral speech perception performances.
Methods
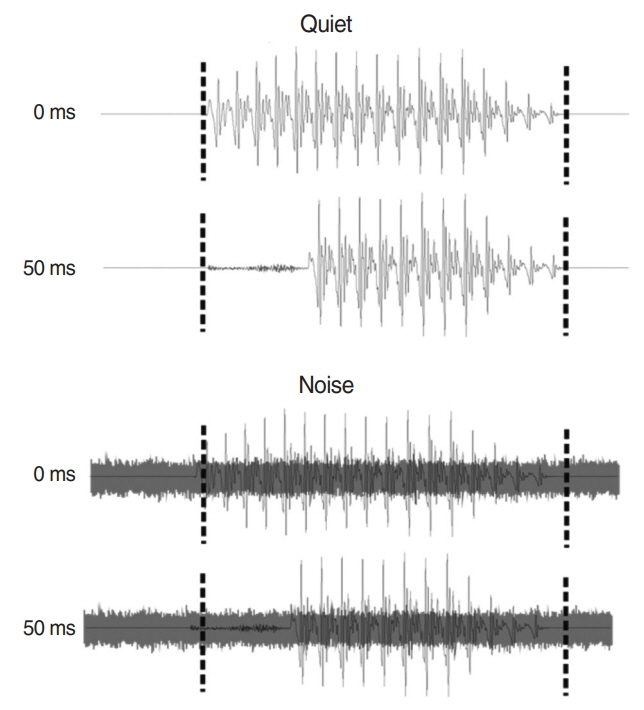
. Ten adult CI users and 15 normal-hearing (NH) people participated in this study. CAEPs were recorded from 64 scalp electrodes in both quiet and noise (signal-to-noise ratio +5 dB) and in passive and active (requiring consonant discrimination) listening. Speech stimulus was synthesized consonant-vowels with VOTs of 0 and 50 ms. N1-P2 amplitudes and latencies were analyzed as a function of listening condition. For the active condition, the P3b also was analyzed. Behavioral measures included a variety of speech perception tasks.
Results
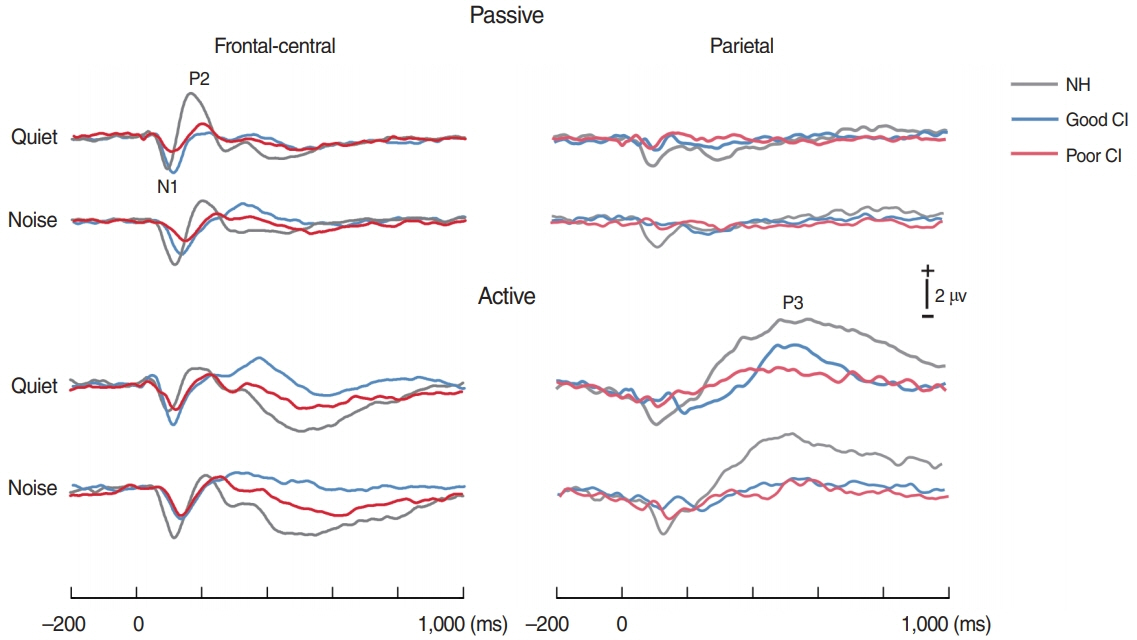
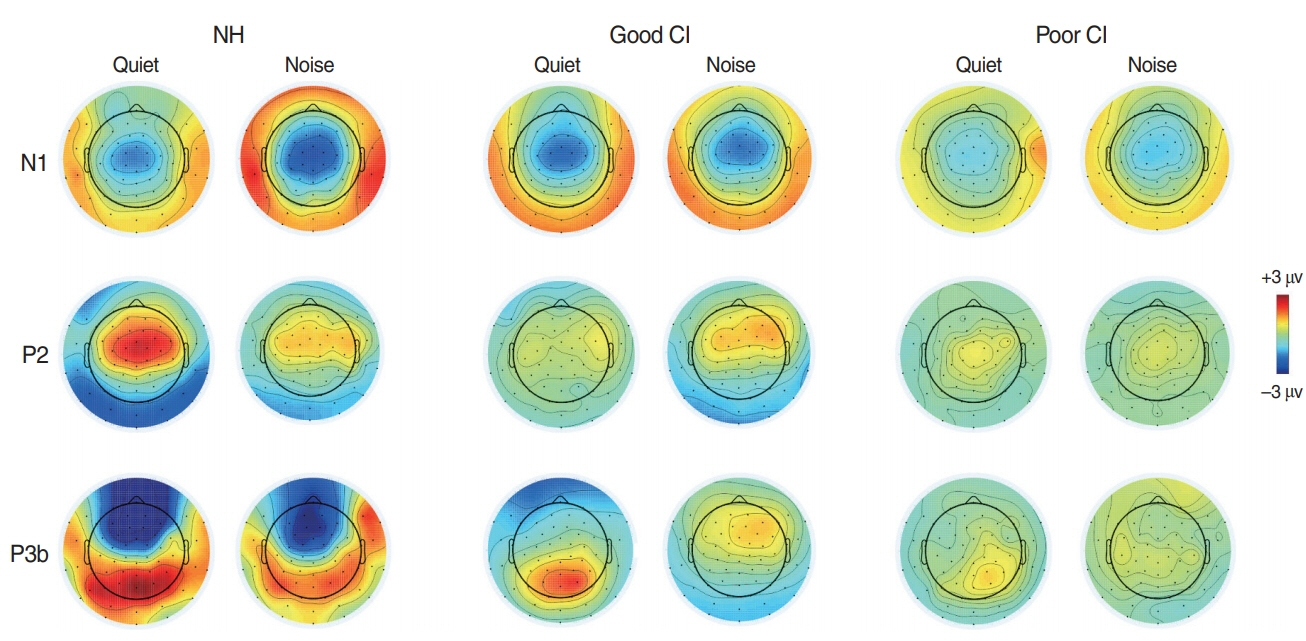
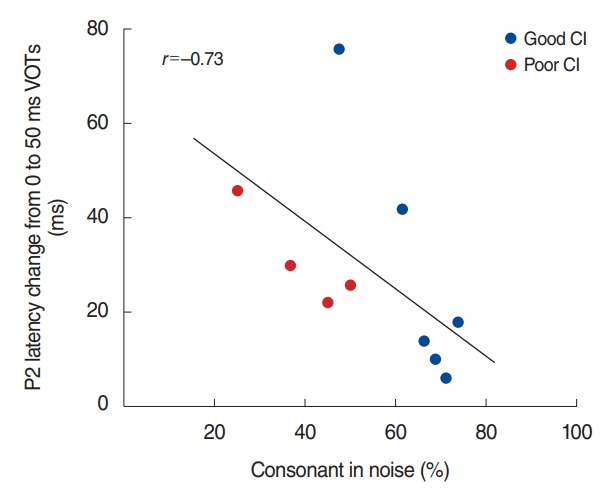
. For good performing CI users, performance in most speech test was lower in the presence of noise masking. N1 and P2 latencies became prolonged with noise masking. The P3b amplitudes were smaller in CI groups compared to NH. The degree of P2 latency change (0 vs. 50 ms VOT) was correlated with consonant perception in noise.
Conclusion
. The effects of noise masking on temporal processing can be reflected in cortical responses in CI users. N1/P2 latencies were more sensitive to noise masking than amplitude measures. Additionally, P2 responses appear to have a better relationship to speech perception in CI users compared to N1.
Figure
Cited by 1 articles
-
Exploring the Neurodynamic Signals of the Deafened Brain: Factors Influencing Cochlear Implant Outcomes
Anu Sharma, Jeong-Sug Kyong
Clin Exp Otorhinolaryngol. 2020;13(3):211-212. doi: 10.21053/ceo.2020.00500.
Reference
-
1. Ponton CW, Don M, Eggermont JJ, Waring MD, Masuda A. Maturation of human cortical auditory function: differences between normal-hearing children and children with cochlear implants. Ear Hear. 1996; Oct. 17(5):430–7.
Article2. Rouger J, Lagleyre S, Fraysse B, Deneve S, Deguine O, Barone P. Evidence that cochlear-implanted deaf patients are better multisensory integrators. Proc Natl Acad Sci U S A. 2007; Apr. 104(17):7295–300.
Article3. Niemitalo-Haapola E, Haapala S, Jansson-Verkasalo E, Kujala T. Background noise degrades central auditory processing in toddlers. Ear Hear. 2015; Nov-Dec. 36(6):e342–51.
Article4. Fu QJ, Nogaki G. Noise susceptibility of cochlear implant users: the role of spectral resolution and smearing. J Assoc Res Otolaryngol. 2005; Mar. 6(1):19–27.
Article5. Hopkins K, Moore BC. The contribution of temporal fine structure to the intelligibility of speech in steady and modulated noise. J Acoust Soc Am. 2009; Jan. 125(1):442–6.
Article6. Won JH, Drennan WR, Rubinstein JT. Spectral-ripple resolution correlates with speech reception in noise in cochlear implant users. J Assoc Res Otolaryngol. 2007; Sep. 8(3):384–92.
Article7. Anderson S, White-Schwoch T, Parbery-Clark A, Kraus N. A dynamic auditory-cognitive system supports speech-in-noise perception in older adults. Hear Res. 2013; Jun. 300:18–32.
Article8. Bidelman GM, Howell M. Functional changes in inter- and intra-hemispheric cortical processing underlying degraded speech perception. Neuroimage. 2016; Jan. 124(Pt A):581–90.
Article9. Zhang C, Lu L, Wu X, Li L. Attentional modulation of the early cortical representation of speech signals in informational or energetic masking. Brain Lang. 2014; Aug. 135:85–95.
Article10. Dimitrijevic A, Pratt H, Starr A. Auditory cortical activity in normal hearing subjects to consonant vowels presented in quiet and in noise. Clin Neurophysiol. 2013; Jun. 124(6):1204–15.
Article11. Parbery-Clark A, Marmel F, Bair J, Kraus N. What subcortical-cortical relationships tell us about processing speech in noise. Eur J Neurosci. 2011; Feb. 33(3):549–57.
Article12. Rao A, Zhang Y, Miller S. Selective listening of concurrent auditory stimuli: an event-related potential study. Hear Res. 2010; Sep. 268(1-2):123–32.
Article13. Fu QJ, Galvin JJ 3rd. Maximizing cochlear implant patients’ performance with advanced speech training procedures. Hear Res. 2008; Aug. 242(1-2):198–208.
Article14. Oba SI, Fu QJ, Galvin JJ 3rd. Digit training in noise can improve cochlear implant users’ speech understanding in noise. Ear Hear. 2011; Sep-Oct. 32(5):573–81.
Article15. Han JH, Zhang F, Kadis DS, Houston LM, Samy RN, Smith ML, et al. Auditory cortical activity to different voice onset times in cochlear implant users. Clin Neurophysiol. 2016; Feb. 127(2):1603–17.
Article16. Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004; Mar. 134(1):9–21.
Article17. Picton TW, Hillyard SA. Human auditory evoked potentials. II. Effects of attention. Electroencephalogr Clin Neurophysiol. 1974; Feb. 36(2):191–9.
Article18. Toscano JC, McMurray B, Dennhardt J, Luck SJ. Continuous perception and graded categorization: electrophysiological evidence for a linear relationship between the acoustic signal and perceptual encoding of speech. Psychol Sci. 2010; Oct. 21(10):1532–40.19. Tremblay K, Ross B. Effects of age and age-related hearing loss on the brain. J Commun Disord. 2007; Jul-Aug. 40(4):305–12.
Article20. Bertoli S, Smurzynski J, Probst R. Effects of age, age-related hearing loss, and contralateral cafeteria noise on the discrimination of small frequency changes: psychoacoustic and electrophysiological measures. J Assoc Res Otolaryngol. 2005; Sep. 6(3):207–22.
Article21. Alain C, Roye A, Salloum C. Effects of age-related hearing loss and background noise on neuromagnetic activity from auditory cortex. Front Syst Neurosci. 2014; Jan. 8:8.
Article22. Shtyrov Y, Kujala T, Ilmoniemi RJ, Naatanen R. Noise affects speechsignal processing differently in the cerebral hemispheres. Neuroreport. 1999; Jul. 10(10):2189–92.
Article23. Sharma M, Purdy SC, Munro KJ, Sawaya K, Peter V. Effects of broadband noise on cortical evoked auditory responses at different loudness levels in young adults. Neuroreport. 2014; Mar. 25(5):312–9.
Article24. Henkin Y, Kileny PR, Hildesheimer M, Kishon-Rabin L. Phonetic processing in children with cochlear implants: an auditory event-related potentials study. Ear Hear. 2008; Apr. 29(2):239–49.
Article25. Henkin Y, Tetin-Schneider S, Hildesheimer M, Kishon-Rabin L. Cortical neural activity underlying speech perception in postlingual adult cochlear implant recipients. Audiol Neurootol. 2009; 14(1):39–53.
Article26. Henkin Y, Yaar-Soffer Y, Steinberg M, Muchnik C. Neural correlates of auditory-cognitive processing in older adult cochlear implant recipients. Audiol Neurootol. 2014; 19 Suppl 1:21–6.
Article27. Groenen PA, Beynon AJ, Snik AF, van den Broek P. Speech-evoked cortical potentials and speech recognition in cochlear implant users. Scand Audiol. 2001; 30(1):31–40.28. Beynon AJ, Snik AF, Stegeman DF, van den Broek P. Discrimination of speech sound contrasts determined with behavioral tests and eventrelated potentials in cochlear implant recipients. J Am Acad Audiol. 2005; Jan. 16(1):42–53.
Article29. Alain C, Campeanu S, Tremblay K. Changes in sensory evoked responses coincide with rapid improvement in speech identification performance. J Cogn Neurosci. 2010; Feb. 22(2):392–403.
Article30. Snyder JS, Pasinski AC, McAuley JD. Listening strategy for auditory rhythms modulates neural correlates of expectancy and cognitive processing. Psychophysiology. 2011; Feb. 48(2):198–207.
Article31. Dimitrijevic A, Smith ML, Kadis DS, Moore DR. Cortical alpha oscillations predict speech intelligibility. Front Hum Neurosci. 2017; Feb. 11:88.
Article32. Choi I, Rajaram S, Varghese LA, Shinn-Cunningham BG. Quantifying attentional modulation of auditory-evoked cortical responses from single-trial electroencephalography. Front Hum Neurosci. 2013; Apr. 7:115.
Article33. Schumann A, Hast A, Hoppe U. Speech performance and training effects in the cochlear implant elderly. Audiol Neurootol. 2014; 19 Suppl 1:45–8.
Article34. Snyder JS, Alain C, Picton TW. Effects of attention on neuroelectric correlates of auditory stream segregation. J Cogn Neurosci. 2006; Jan. 18(1):1–13.
Article35. Billings CJ, Bennett KO, Molis MR, Leek MR. Cortical encoding of signals in noise: effects of stimulus type and recording paradigm. Ear Hear. 2011; Feb. 32(1):53–60.
Article36. Tremblay KL, Ross B, Inoue K, McClannahan K, Collet G. Is the auditory evoked P2 response a biomarker of learning. Front Syst Neurosci. 2014; Feb. 8:28.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Behavioral Perception and Neural Correlates of Categorical Perception in Cochlear Implant Users
- Evaluation of the ClearVoice Strategy in Adults Using HiResolution Fidelity 120 Sound Processing
- A Review of Music Perception with Cochlear Implantation
- Auditory Cortical Temporal Processing and Hemispheric Asymmetry Revealed by N1 Dipole Source Activity in Adult Cochlear Implant Users
- Definition and Clinical Implication of Temporal Fine Structure