Anesth Pain Med.
2020 Jul;15(3):291-296. 10.17085/apm.20004.
Effects of tranexamic acid on the activity of glutamate transporter EAAT3
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Seoul National University Bundang Hospital, Seongnam, Korea
- 2Department of Anesthesiology and Pain Medicine, ThanQ Seoul Thyroid-Head & Neck Surgery Center, Seoul, Korea
- KMID: 2504890
- DOI: http://doi.org/10.17085/apm.20004
Abstract
- Background
Tranexamic acid (TXA) is the most widely used hemostatic agent in surgical patients. However, when used in a high dose, it could cause a seizure in the postoperative period. The exact effector mechanism behind the seizure triggering remains unknown. Therefore, the authors investigated the effects of TXA on the activity of glutamate transporter type 3 (excitatory amino acid transporter 3; EAAT3), which is the main neuronal glutamate transporter type.
Methods
EAAT3 was expressed in Xenopus laevis oocytes through mRNA injection. Oocytes were incubated with diluted tranexamic acid for 72 h. Two-electrode voltage clamping was used to measure membrane currents before, during, and after applying 30 M L-glutamate. Responses were quantified by integrating the current traces and reported in microcoulombs (C). Results were presented as mean SEM.
Results
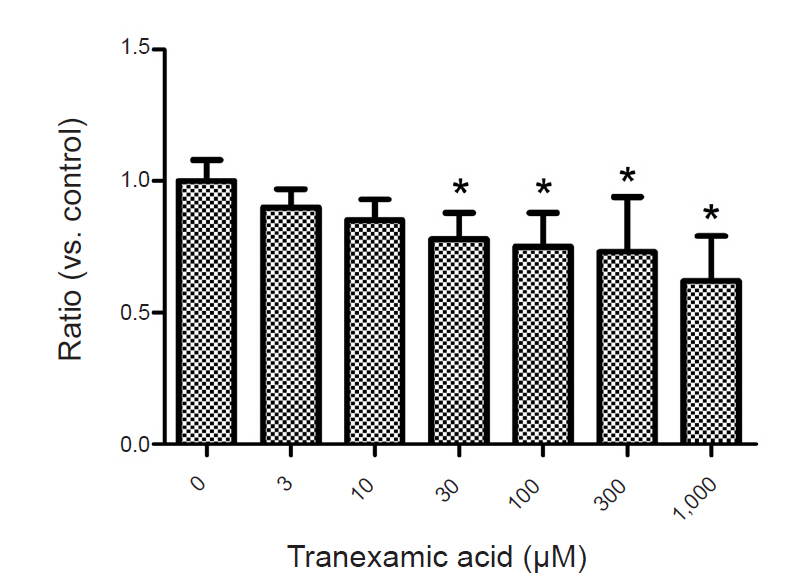
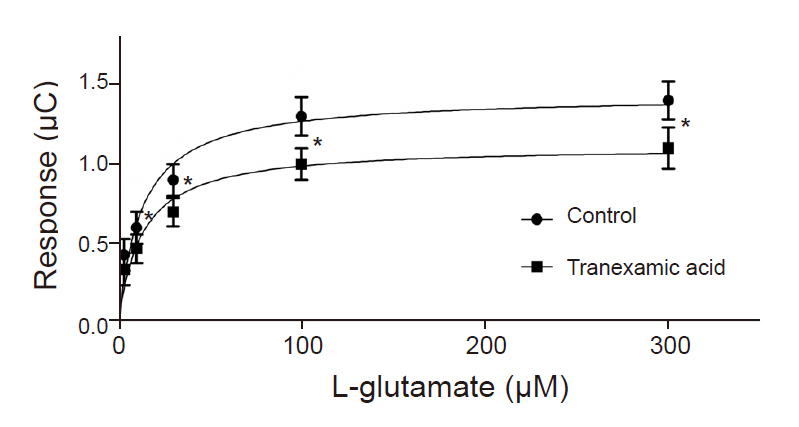
TXA (30 to 1,000 M) significantly decreased EAAT3 activity. Our kinetic study showed that Vmax was significantly decreased in the TXA group compared with the control group (1.1 0.1 vs. 1.4 0.1 C, n = 18–23, P = 0.043), but the Km did not significantly change (12.7 3.9 M for TXA vs. 12.8 3.8 for control, n = 18–23, P = 0.986).
Conclusions
Our results suggest that TXA attenuates EAAT3 activity, which may explain its proconvulsant effect.
Keyword
Figure
Reference
-
1. Kalavrouziotis D, Voisine P, Mohammadi S, Dionne S, Dagenais F. High-dose tranexamic acid is an independent predictor of early seizure after cardiopulmonary bypass. Ann Thorac Surg. 2012; 93:148–54.2. Merriman B, Mayson K, Sawka A, Akagami R, Flexman AM. Postoperative seizure in a neurosurgical patient: should tranexamic acid be on the differential? Can J Anaesth. 2013; 60:506–7.3. Kratzer S, Irl H, Mattusch C, Bürge M, Kurz J, Kochs E, et al. Tranexamic acid impairs γ-aminobutyric acid receptor type A-mediated synaptic transmission in the murine amygdala: a potential mechanism for drug-induced seizures? Anesthesiology. 2014; 120:639–49.4. Sepkuty JP, Cohen AS, Eccles C, Rafiq A, Behar K, Ganel R, et al. A neuronal glutamate transporter contributes to neurotransmitter GABA synthesis and epilepsy. J Neurosci. 2002; 22:6372–9.5. Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001; 65:1–105.6. Beart PM, O'Shea RD. Transporters for L-glutamate: an update on their molecular pharmacology and pathological involvement. Br J Pharmacol. 2007; 150:5–17.7. Maragakis NJ, Rothstein JD. Glutamate transporters in neurologic disease. Arch Neurol. 2001; 58:365–70.8. Sheldon AL, Robinson MB. The role of glutamate transporters in neurodegenerative diseases and potential opportunities for intervention. Neurochem Int. 2007; 51:333–55.9. Do SH, Kamatchi GL, Washington JM, Zuo Z. Effects of volatile anesthetics on glutamate transporter, excitatory amino acid transporter type 3: the role of protein kinase C. Anesthesiology. 2002; 96:1492–7.10. Guo J, Gao X, Ma Y, Lv H, Hu W, Zhang S, et al. Different dose regimes and administration methods of tranexamic acid in cardiac surgery: a meta-analysis of randomized trials. BMC Anesthesiol. 2019; 19:129.11. Manji RA, Grocott HP, Leake J, Ariano RE, Manji JS, Menkis AH, et al. Seizures following cardiac surgery: the impact of tranexamic acid and other risk factors. Can J Anaesth. 2012; 59:6–13.12. Zhang Y, Bai Y, Chen M, Zhou Y, Yu X, Zhou H, et al. The safety and efficiency of intravenous administration of tranexamic acid in coronary artery bypass grafting (CABG): a meta-analysis of 28 randomized controlled trials. BMC Anesthesiol. 2019; 19:104.13. Mathews GC, Diamond JS. Neuronal glutamate uptake contributes to GABA synthesis and inhibitory synaptic strength. J Neurosci. 2003; 23:2040–8.14. Lecker I, Wang DS, Romaschin AD, Peterson M, Mazer CD, Orser BA. Tranexamic acid concentrations associated with human seizures inhibit glycine receptors. J Clin Invest. 2012; 122:4654–66.15. Na HS, Park HP, Kim CS, Do SH, Zuo Z, Kim CS. 17β-Estradiol attenuates the activity of the glutamate transporter type 3 expressed in Xenopus oocytes. Eur J Pharmacol. 2012; 676:20–5.16. Yun JY, Kim JH, Kim HK, Lim YJ, Do SH, Zuo Z. Effects of intravenous anesthetics on the activity of glutamate transporter EAAT3 expressed in Xenopus oocytes: evidence for protein kinase C involvement. Eur J Pharmacol. 2006; 531:133–9.17. Lecker I, Wang DS, Whissell PD, Avramescu S, Mazer CD, Orser BA. Tranexamic acid-associated seizures: causes and treatment. Ann Neurol. 2016; 79:18–26.18. Furtmüller R, Schlag MG, Berger M, Hopf R, Huck S, Sieghart W, et al. Tranexamic acid, a widely used antifibrinolytic agent, causes convulsions by a gamma-aminobutyric acid(A) receptor antagonistic effect. J Pharmacol Exp Ther. 2002; 301:168–73.19. Kim JH, Do SH, Kim YL, Zuo Z. Effects of chronic exposure to ethanol on glutamate transporter EAAT3 expressed in Xenopus oocytes: evidence for protein kinase C involvement. Alcohol Clin Exp Res. 2005; 29:2046–52.20. Kim JH, Lim YJ, Ro YJ, Min SW, Kim CS, Do SH, et al. Effects of ethanol on the rat glutamate excitatory amino acid transporter type 3 expressed in Xenopus oocytes: role of protein kinase C and phosphatidylinositol 3-kinase. Alcohol Clin Exp Res. 2003; 27:1548–53.21. Shin HJ, Ryu JH, Kim ST, Zuo Z, Do SH. Caffeine-induced inhibition of the activity of glutamate transporter type 3 expressed in Xenopus oocytes. Toxicol Lett. 2013; 217:143–8.22. Yoon HJ, Lim YJ, Zuo Z, Hur W, Do SH. Nicotine decreases the activity of glutamate transporter type 3. Toxicol Lett. 2014; 225:147–52.23. Ryu J, Do SH, Lee N. Remifentanil increases the activity of the glutamate transporter, EAAC1, expressed in Xenopus oocytes. Anesth Pain Med. 2008; 3:264–9.24. Ryu JH, Kim CS, Do SH, Park HS. Effect of propofol on oxidative stress-attenuated glutamate transporter EAAT4 activity. Anesth Pain Med. 2011; 6:225–30.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of clonidine on the activity of the rat glutamate transporter EAAT3 expressed in Xenopus oocytes
- Effects of Tianeptine on The Activity of Glutamate Transporter EAAT3 Expressed in Xenopus Oocytes
- Is irrational use of tranexamic acid justified in anesthesia practice?
- Treatment Effect of Tranexamic Acid in Plasma D-dimer Level Elevated Anti-histamine Resistant Chronic Urticaria Patients
- Melasma Showing Response to Combination Therapy with Oral Tranexamic Acid and the Q-Switched Nd:YAG Laser