Ann Surg Treat Res.
2020 Aug;99(2):82-89. 10.4174/astr.2020.99.2.82.
The prognoses and postoperative outcomes of patients with both colorectal cancer and liver cirrhosis based on a nationwide cohort in Korea
- Affiliations
-
- 1Department of Public Health Sciences and Institute of Health and Environment, Seoul National University, Seoul, Korea
- 2Department of Surgery, Dongnam Institute of Radiological and Medical Sciences, Busan, Korea
- 3Interdisciplinary Program in Bioinformatics, Seoul National University, Seoul, Korea
- 4Department of Surgery, Seoul National University College of Medicine, Seoul, Korea
- 5Department of Surgery, Seoul National University Hospital Healthcare System Gangnam Center, Seoul, Korea
- 6Department of Surgery, Chung-Ang University College of Medicine, Seoul, Korea
- KMID: 2504820
- DOI: http://doi.org/10.4174/astr.2020.99.2.82
Abstract
- Purpose
The management of patients with colorectal cancer (CRC) who have liver cirrhosis (LC) requires a thorough understanding of both diseases; however, the prognoses and postoperative outcomes of such patients remain understudied. We investigated the effect of LC on surgical and oncologic outcomes in patients with CRC, and identified prognostic factors.
Methods
We analyzed 453 patients with CRC and LC (CRC-LC group), 906 with CRC only (CRC group), 906 with LC only (LC group), and 1,812 healthy subjects using health insurance claim data (2008–2013).
Results
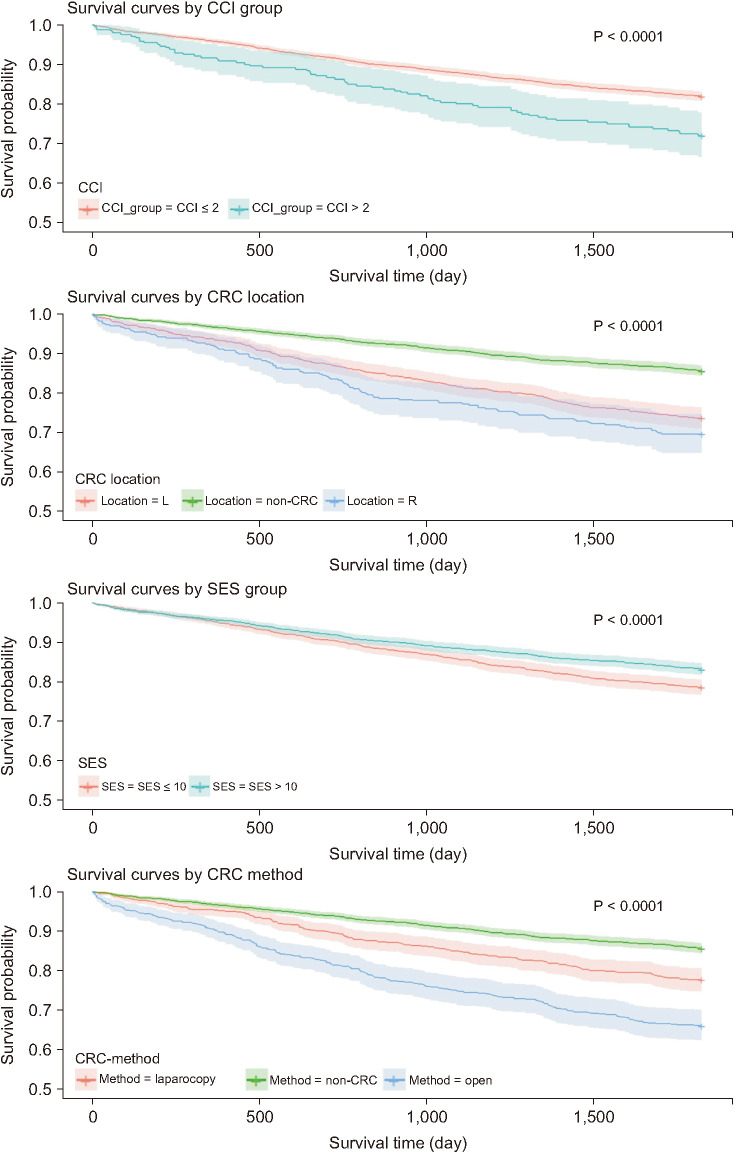
The CRC-LC group had a higher frequency of intensive care unit admission than the CRC group; there were no differences between the 2 groups in terms of early and late postoperative small bowel obstruction and incisional hernia. However, the 30-day, 60-day, and 90-day mortality rates were all significantly higher in the CRC-LC group. The higher Charlson comorbidity index (hazard ratio [HR], 1.127) and the lower socioeconomic status (HR, 0.985) were significant worse predictors of 5-year survival. Patients with underlying LC had a significantly higher HR in both the advanced CRC (HR, 1.858) and nonadvanced CRC (HR, 1.799) subgroups. However, the nonadvanced CRC subgroup showed a lower HR than the LC group (HR, 0.730).
Conclusion
Patients with CRC who had underlying LC had a lower survival rate than did those without LC, although the incidence rates of postoperative complications were not significantly different. The presence of LC was associated with a significantly lower survival rate regardless of CRC presence.
Figure
Cited by 1 articles
-
Real-world survival after colorectal surgery for malignancy in Korean patients with chronic kidney disease: an analysis of Korean healthcare big data, 2002–2019
Inho Song, Hyeryeong Nam, Bora Lee, Byung Kwan Park, Jeong-ki Kim, Seung-Bum Ryoo, Kyu Joo Park, Eon Chul Han
Ann Surg Treat Res. 2023;105(5):281-289. doi: 10.4174/astr.2023.105.5.281.
Reference
-
1. Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014; 383:1749–1761. PMID: 24480518.2. Han EC, Ryoo SB, Park JW, Yi JW, Oh HK, Choe EK, et al. Oncologic and surgical outcomes in colorectal cancer patients with liver cirrhosis: a propensity-matched study. PLoS One. 2017; 12:e0178920. PMID: 28586376.3. Friedman LS. Surgery in the patient with liver disease. Trans Am Clin Climatol Assoc. 2010; 121:192–204. PMID: 20697561.4. Nguyen GC, Correia AJ, Thuluvath PJ. The impact of cirrhosis and portal hypertension on mortality following colorectal surgery: a nationwide, population-based study. Dis Colon Rectum. 2009; 52:1367–1374. PMID: 19617746.5. Lopez-Delgado JC, Ballus J, Esteve F, Betancur-Zambrano NL, Corral-Velez V, Manez R, et al. Outcomes of abdominal surgery in patients with liver cirrhosis. World J Gastroenterol. 2016; 22:2657–2667. PMID: 26973406.6. Ziser A, Plevak DJ, Wiesner RH, Rakela J, Offord KP, Brown DL. Morbidity and mortality in cirrhotic patients undergoing anesthesia and surgery. Anesthesiology. 1999; 90:42–53. PMID: 9915311.7. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424. PMID: 30207593.8. Baek Y, Yi M. Factors influencing quality of life during chemotherapy for colorectal cancer patients in South Korea. J Korean Acad Nurs. 2015; 45:604–612. PMID: 26364535.9. Sorensen HT, Friis S, Olsen JH, Thulstrup AM, Mellemkjaer L, Linet M, et al. Risk of liver and other types of cancer in patients with cirrhosis: a nationwide cohort study in Denmark. Hepatology. 1998; 28:921–925. PMID: 9755226.10. Lee YH, Han K, Ko SH, Ko KS, Lee KU. Taskforce Team of Diabetes Fact Sheet of the Korean Diabetes Association. Data analytic process of a nationwide population-based study using national health information database established by national health insurance service. Diabetes Metab J. 2016; 40:79–82. PMID: 26912157.11. Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005; 43:1130–1139. PMID: 16224307.12. R Core Team. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing;2013. Available online at https://www.R-project.org/.13. An J, Won S, Lutz SM, Hecker J, Lange C. Effect of population stratification on SNP-by-environment interaction. Genet Epidemiol. 2019; 43:1046–1055. PMID: 31429121.14. Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS Med. 2014; 11:e1001624. PMID: 24691105.15. Bruix J, Reig M, Sherman M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology. 2016; 150:835–853. PMID: 26795574.16. Shim JI, Kim Y, Han MA, Lee HY, Choi KS, Jun JK, et al. Results of colorectal cancer screening of the national cancer screening program in Korea, 2008. Cancer Res Treat. 2010; 42:191–198. PMID: 21253320.17. Csikesz NG, Nguyen LN, Tseng JF, Shah SA. Nationwide volume and mortality after elective surgery in cirrhotic patients. J Am Coll Surg. 2009; 208:96–103. PMID: 19228510.18. Wu CC, Hsu TW, Chang CM, Yu CH, Lee CC. Age-adjusted Charlson comorbidity index scores as predictor of survival in colorectal cancer patients who underwent surgical resection and chemoradiation. Medicine (Baltimore). 2015; 94:e431. PMID: 25590852.19. Chang CM, Yin WY, Wei CK, Wu CC, Su YC, Yu CH, et al. Adjusted age-adjusted Charlson comorbidity index score as a risk measure of perioperative mortality before cancer surgery. PLoS One. 2016; 11:e0148076. PMID: 26848761.20. Laor A, Tal S, Guller V, Zbar AP, Mavor E. The Charlson Comorbidity Index (CCI) as a mortality predictor after surgery in elderly patients. Am Surg. 2016; 82:22–27. PMID: 26802847.21. Utsunomiya T, Saitsu H, Saku M, Yoshida K, Matsumata T, Shimada M, et al. Rare occurrence of colorectal cancer metastasis in livers infected with hepatitis B or C virus. Am J Surg. 1999; 177:279–281. PMID: 10326842.22. Song E, Chen J, Ou Q, Su F. Rare occurrence of metastatic colorectal cancers in livers with replicative hepatitis B infection. Am J Surg. 2001; 181:529–533. PMID: 11513779.23. Schrag D, Cramer LD, Bach PB, Begg CB. Age and adjuvant chemotherapy use after surgery for stage III colon cancer. J Natl Cancer Inst. 2001; 93:850–857. PMID: 11390534.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Propofol-based intravenous anesthesia is associated with improved survival outcomes after major cancer surgery: a nationwide cohort study in South Korea
- The Significance of Postoperative Serial CEA Levels in Recurrent Colorectal Cancer
- Outcomes of liver resection in patients with colorectal liver metastases by laparoscopic or open surgery
- Outcomes of a Hepatic Resection for Colorectal-Carcinoma Liver Metastases
- Living donor liver transplantation for unresectable colorectal liver metastasis: Report of a case with 13-year follow-up without recurrence