J Korean Med Sci.
2020 Jul;35(29):e238. 10.3346/jkms.2020.35.e238.
Proarrhythmogenic Effect of the L532P and N588K KCNH2 Mutations in the Human Heart Using a 3D Electrophysiological Model
- Affiliations
-
- 1School of Computing, Telkom University, Bandung, Jawa Barat, Indonesia
- 2Research Center of Human Centric Engineering (HUMIC), Telkom University, Bandung, Jawa Barat, Indonesia
- 3Department of IT Convergence Engineering, Kumoh National Institute of Technology, Gumi, Korea
- KMID: 2504700
- DOI: http://doi.org/10.3346/jkms.2020.35.e238
Abstract
- Background
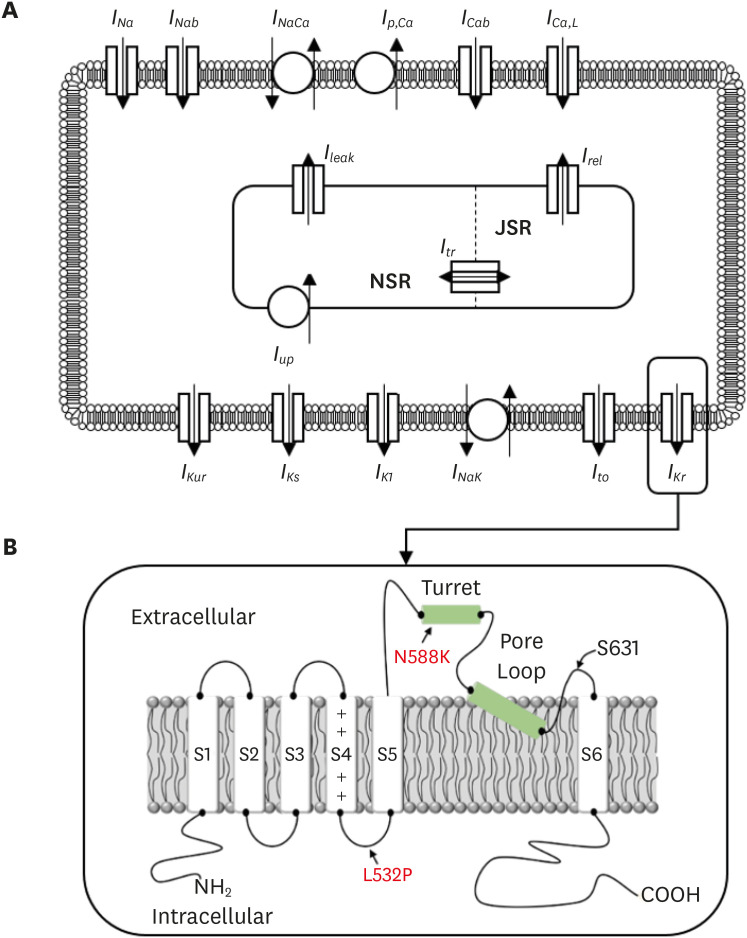
Atrial arrhythmia is a cardiac disorder caused by abnormal electrical signaling and transmission, which can result in atrial fibrillation and eventual death. Genetic defects in ion channels can cause myocardial repolarization disorders. Arrhythmia-associated gene mutations, including KCNH2 gene mutations, which are one of the most common genetic disorders, have been reported. This mutation causes abnormal QT intervals by a gain of function in the rapid delayed rectifier potassium channel (IKr). In this study, we demonstrated that mutations in the KCNH2 gene cause atrial arrhythmia.
Methods
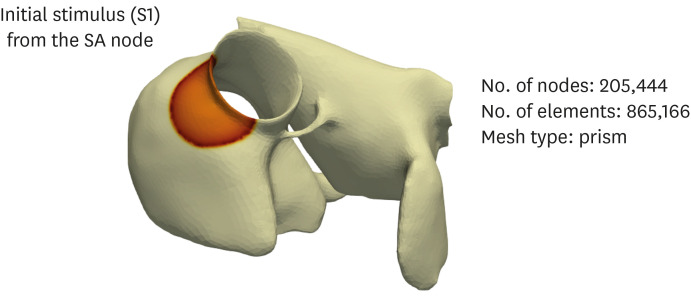
The N588K and L532P mutations were induced in the Courtemanche-Ramirez-Nattel (CRN) cell model, which was subjected to two-dimensional and three-dimensional simulations to compare the electrical conduction patterns of the wild-type and mutant-type genes.
Results
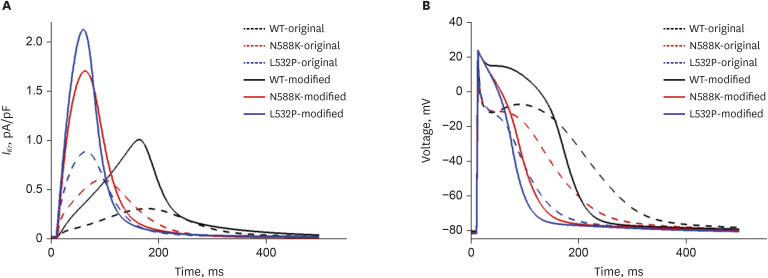
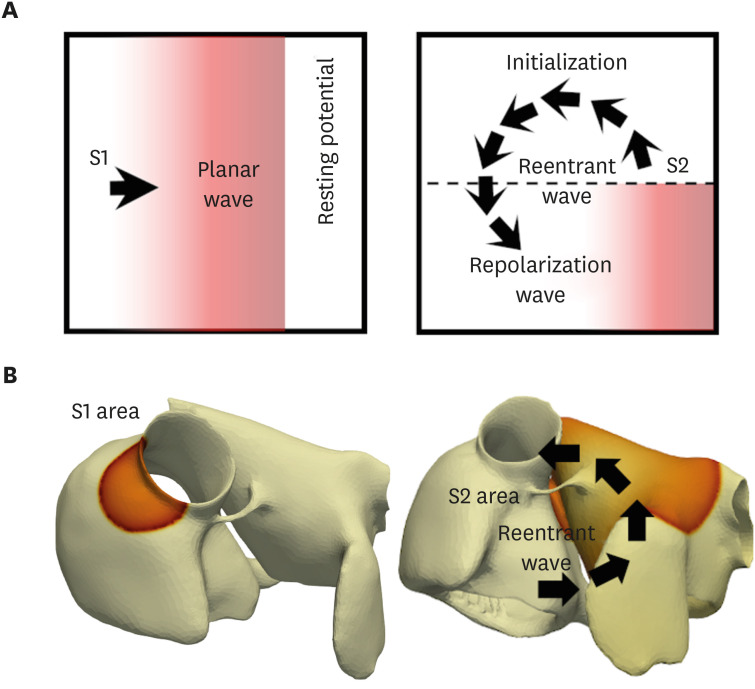
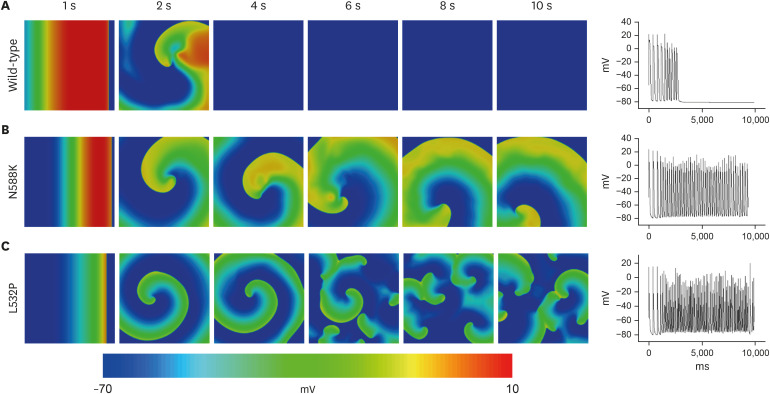
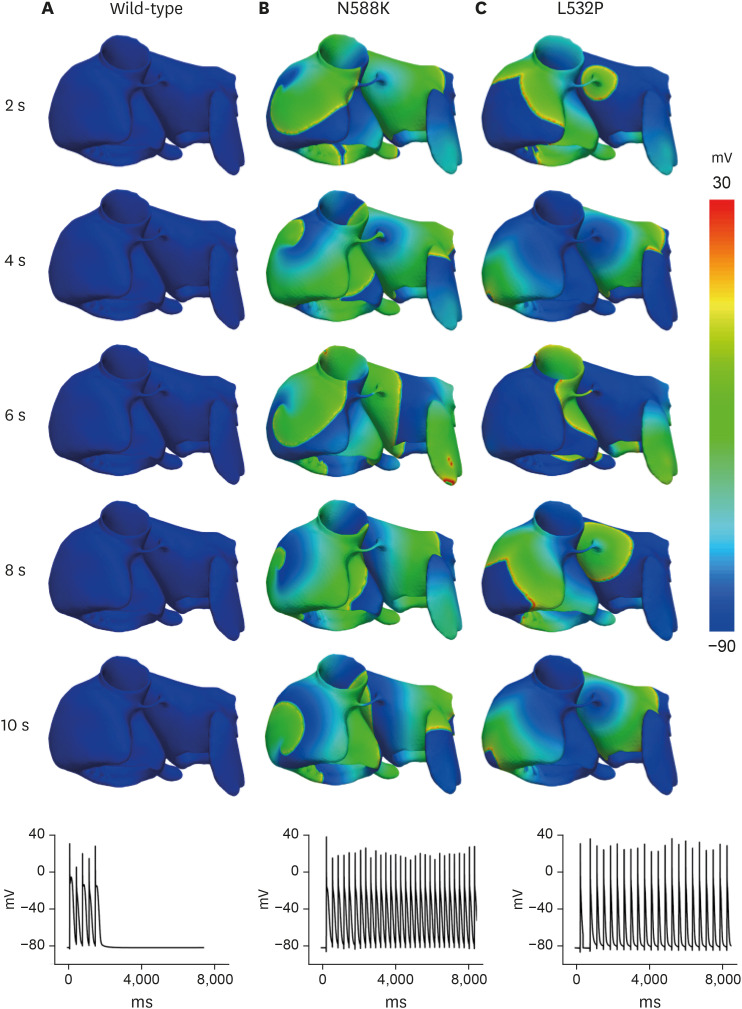
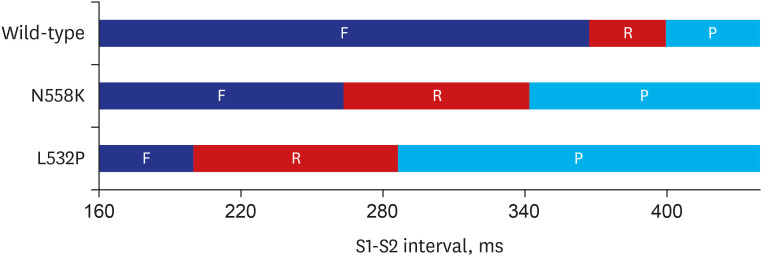
In contrast to the early self-termination of the wild-type conduction waveforms, the conduction waveform of the mutant-type retained the reentrant wave (N588K) and caused a spiral break-up, resulting in irregular wave generation (L532P).
Conclusion
The present study confirmed that the KCNH2 gene mutation increases the vulnerability of the atrial tissue for arrhythmia.
Figure
Reference
-
1. Hsiao PY, Tien HC, Lo CP, Juang JM, Wang YH, Sung RJ. Gene mutations in cardiac arrhythmias: a review of recent evidence in ion channelopathies. Appl Clin Genet. 2013; 6:1–13. PMID: 23837003.2. Wilde AA, Bezzina CR. Genetics of cardiac arrhythmias. Heart. 2005; 91(10):1352–1358. PMID: 16162633.
Article3. Chen PS, Garfinkel A, Weiss JN, Karagueuzian HS. CHEN PS. Garfinkel A, Weiss JN, Karagueuzian HS. Spirals, chaos, and new mechanisms of wave propagation. Pacing Clin Electrophysiol. 1997; 20(2):414–421. PMID: 9058845.4. Kannel WB, Benjamin EJ. Status of the epidemiology of atrial fibrillation. Med Clin North Am. 2008; 92(1):17–40. PMID: 18060995.
Article5. Stewart S, Hart CL, Hole DJ, McMurray JJ. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med. 2002; 113(5):359–364. PMID: 12401529.
Article6. Brugada R, Hong K, Dumaine R, Cordeiro J, Gaita F, Borggrefe M, et al. Sudden death associated with short-QT syndrome linked to mutations in HERG. Circulation. 2004; 109(1):30–35. PMID: 14676148.
Article7. Gussak I, Brugada P, Brugada J, Wright RS, Kopecky SL, Chaitman BR, et al. Idiopathic short QT interval: a new clinical syndrome? Cardiology. 2000; 94(2):99–102. PMID: 11173780.8. Giustetto C, Di Monte F, Wolpert C, Borggrefe M, Schimpf R, Sbragia P, et al. Short QT syndrome: clinical findings and diagnostic-therapeutic implications. Eur Heart J. 2006; 27(20):2440–2447. PMID: 16926178.
Article9. Hong K, Bjerregaard P, Gussak I, Brugada R. Short QT syndrome and atrial fibrillation caused by mutation in KCNH2. J Cardiovasc Electrophysiol. 2005; 16(4):394–396. PMID: 15828882.
Article10. Zhang YH, Colenso CK, Sessions RB, Dempsey CE, Hancox JC. The hERG K(+) channel S4 domain L532P mutation: characterization at 37°C. Biochim Biophys Acta. 2011; 1808(10):2477–2487. PMID: 21777565.
Article11. Sanguinetti MC, Jiang C, Curran ME, Keating MT. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell. 1995; 81(2):299–307. PMID: 7736582.12. Trudeau MC, Warmke JW, Ganetzky B, Robertson GA. HERG, a human inward rectifier in the voltage-gated potassium channel family. Science. 1995; 269(5220):92–95. PMID: 7604285.
Article13. Hassel D, Scholz EP, Trano N, Friedrich O, Just S, Meder B, et al. CLINICAL PERSPECTIVE. Circulation. 2008; 117(7):866–875. PMID: 18250272.14. Vandenberg JI, Walker BD, Campbell TJ. HERG K+ channels: friend and foe. Trends Pharmacol Sci. 2001; 22(5):240–246. PMID: 11339975.
Article15. Adeniran I, El Harchi A, Hancox JC, Zhang H. Proarrhythmia in KCNJ2-linked short QT syndrome: insights from modelling. Cardiovasc Res. 2012; 94(1):66–76. PMID: 22308236.
Article16. Imaniastuti R, Lee HS, Kim N, Youm JB, Shim EB, Lim KM. Computational prediction of proarrhythmogenic effect of the V241F KCNQ1 mutation in human atrium. Prog Biophys Mol Biol. 2014; 116(1):70–75. PMID: 25230101.
Article17. Zulfa I, Shim EB, Song KS, Lim KM. Computational simulations of the effects of the G229D KCNQ1 mutation on human atrial fibrillation. J Physiol Sci. 2016; 66(5):407–415. PMID: 26922794.
Article18. Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952; 117(4):500–544. PMID: 12991237.
Article19. Courtemanche M, Ramirez RJ, Nattel S. Ionic mechanisms underlying human atrial action potential properties: insights from a mathematical model. Am J Physiol. 1998; 275(1):H301–21. PMID: 9688927.
Article20. Jacquemet V, Virag N, Ihara Z, Dang L, Blanc O, Zozor S, et al. Study of unipolar electrogram morphology in a computer model of atrial fibrillation. J Cardiovasc Electrophysiol. 2003; 14(10 Suppl):S172–S179. PMID: 14760921.
Article21. McPate MJ, Duncan RS, Milnes JT, Witchel HJ, Hancox JC. The N588K-HERG K+ channel mutation in the ‘short QT syndrome’: mechanism of gain-in-function determined at 37 degrees C. Biochem Biophys Res Commun. 2005; 334(2):441–449. PMID: 16011830.22. Loewe A, Wilhelms M, Fischer F, Scholz EP, Dössel O, Seemann G. Arrhythmic potency of human ether-a-go-go-related gene mutations L532P and N588K in a computational model of human atrial myocytes. Europace. 2014; 16(3):435–443. PMID: 24569898.23. Hwang M, Kwon SS, Wi J, Park M, Lee HS, Park JS, et al. Virtual ablation for atrial fibrillation in personalized in-silico three-dimensional left atrial modeling: comparison with clinical catheter ablation. Prog Biophys Mol Biol. 2014; 116(1):40–47. PMID: 25261813.
Article24. Xie F, Qu Z, Yang J, Baher A, Weiss JN, Garfinkel A. A simulation study of the effects of cardiac anatomy in ventricular fibrillation. J Clin Invest. 2004; 113(5):686–693. PMID: 14991066.
Article25. Jeong DU, Lim KM. Influence of the KCNQ1 S140G mutation on human ventricular arrhythmogenesis and pumping performance: simulation study. Front Physiol. 2018; 9:926. PMID: 30108508.
Article26. Adeniran I, Hancox JC, Zhang H. In silico investigation of the short QT syndrome, using human ventricle models incorporating electromechanical coupling. Front Physiol. 2013; 4:166. PMID: 23847545.
Article27. Kamiya K, Mitcheson JS, Yasui K, Kodama I, Sanguinetti MC. Open channel block of HERG K(+) channels by vesnarinone. Mol Pharmacol. 2001; 60(2):244–253. PMID: 11455010.
Article28. Volberg WA, Koci BJ, Su W, Lin J, Zhou J. Blockade of human cardiac potassium channel human ether-a-go-go-related gene (HERG) by macrolide antibiotics. J Pharmacol Exp Ther. 2002; 302(1):320–327. PMID: 12065733.
Article29. Dumaine R, Roy ML, Brown AM. Blockade of HERG and Kv1.5 by ketoconazole. J Pharmacol Exp Ther. 1998; 286(2):727–735. PMID: 9694927.30. Roy M, Dumaine R, Brown AM. HERG, a primary human ventricular target of the nonsedating antihistamine terfenadine. Circulation. 1996; 94(4):817–823. PMID: 8772706.
Article31. Heikhmakhtiar AK, Rasyidin FA, Lim KM. V241F KCNQ1 mutation shortens electrical wavelength and reduces ventricular pumping capabilities: a simulation study with an electro-mechanical model. Front Phys. 2018; 6:147.
Article32. Yuniarti AR, Setianto F, Marcellinus A, Hwang HJ, Choi SW, Trayanova N, et al. Effect of KCNQ1 G229D mutation on cardiac pumping efficacy and reentrant dynamics in ventricles: computational study. Int J Numer Methods Biomed Eng. 2018; 34(6):e2970.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Research Progress of Three-Dimensional Bioprinting Artificial Cardiac Tissue
- A novel KCNH2 frameshift mutation (c.46delG) associated with high risk of sudden death in a family with congenital long QT syndrome type 2
- Expression of the IKr components KCNH2 (rERG) and KCNE2 (rMiRP1) during late rat heart development
- Clinical Applications of Three-Dimensional Printing in Cardiovascular Disease
- Clinical Application of Solid Model Based on Trabecular Tibia Bone CT Images Created by 3D Printer