J Korean Neurosurg Soc.
2020 Jul;63(4):504-512. 10.3340/jkns.2019.0205.
Correlation between the Position of the Pituitary Stalk as Determined by Diffusion Tensor Imaging and Its Location as Determined at the Time of Surgical Resection of Pituitary Adenomas
- Affiliations
-
- 1Department of Neurosurgery, The First Medical Center, PLA General Hospital, Beijing, China
- KMID: 2504645
- DOI: http://doi.org/10.3340/jkns.2019.0205
Abstract
Objective
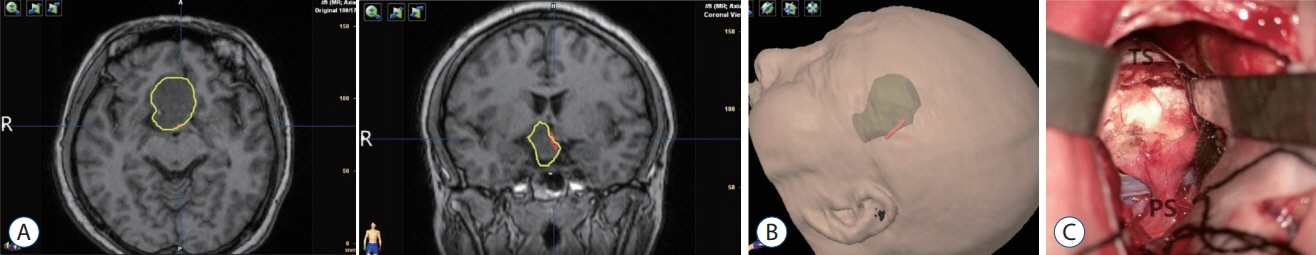
: An important factor during pituitary adenoma surgery is to preserve pituitary stalk (PS) as this plays a role in reduction of the risk of postoperative diabetes insipidus. The hypothalamic-hypophyseal tract (HHT) projects through the PS to the posterior pituitary gland. To reconstruct white matter fiber pathways, methods like diffusion tensor imaging (DTI) tractography have been widely used. In this report we attempted to predict the position of PS using DTI tractography and to assess its intraoperative correlation during surgery of pituitary adenomas.
Methods
: DTI tractography was used to tract the HHT in nine patients before craniotomy for pituitary adenomas. The DTI location of the HHT was compared with the PS position identified at the time of surgery. DTI fiber tracking was carried out in nine patients prior to the planned craniotomy for pituitary adenomas. In one patient, the PS could not be identified during the surgery. In the other eight patients, a comparison was made between the location of the HHT identified by DTI and the position of the PS visualized at the time of surgery.
Results
: The position of the HHT identified by DTI showed consistency with the intraoperative position of the PS in seven patients (88.9% concordance).
Conclusion
: This study shows that DTI can identify the position of the HHT and thus the position of the PS with a high degree of reliability.
Figure
Reference
-
References
1. Ahn JY, Jung JY, Kim J, Lee KS, Kim SH. How to overcome the limitations to determine the resection margin of pituitary tumours with lowfield intra-operative MRI during trans-sphenoidal surgery: usefulness of Gadolinium-soaked cotton pledgets. Acta Neurochir (Wien). 150:763–771. discussion 771, 2008. 2008.
Article2. Albayrak B, Samdani AF, Black PM. Intra-operative magnetic resonance imaging in neurosurgery. Acta Neurochir (Wien). 146:543–556. discussion 557, 2004.
Article3. Ammirati M, Wei L, Ciric I. Short-term outcome of endoscopic versus microscopic pituitary adenoma surgery: a systematic review and metaanalysis. J Neurol Neurosurg Psychiatry. 84:843–849. 2013.
Article4. Araki Y, Ashikaga R, Takahashi S, Ueda J, Ishida O. High signal intensity of the infundibular stalk on fluid-attenuated inversion recovery MR. AJNR Am J Neuroradiol. 18:89–93. 1997.5. Banaszek A, Bladowska J, Pokryszko-Dragan A, Podemski R, Sąsiadek MJ. Evaluation of the degradation of the selected projectile, commissural and association white matter tracts within normal appearing white matter in patients with multiple sclerosis using diffusion tensor MR imaging - a preliminary Study. Pol J Radiol. 80:457–463. 2015.
Article6. Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 111:209–219. 1996.
Article7. Borkar SA, Garg A, Mankotia DS, Joseph SL, Suri A, Kumar R, et al. Prediction of facial nerve position in large vestibular schwannomas using diffusion tensor imaging tractography and its intraoperative correlation. Neurol India. 64:965–970. 2016.
Article8. Chen F, Chen L, Li W, Li L, Xu X, Li W, et al. Pre-operative declining proportion of fractional anisotropy of trigeminal nerve is correlated with the outcome of micro-vascular decompression surgery. BMC Neurol. 16:106. 2016.
Article9. Chen X, Xu BN, Meng X, Zhang J, Yu X, Zhou D. Dual-room 1.5-T intraoperative magnetic resonance imaging suite with a movable magnet: implementation and preliminary experience. Neurosurg Rev. 35:95–109. discussion 109-110. 2012.
Article10. Cho CH, Barkhoudarian G, Hsu L, Bi WL, Zamani AA, Laws ER. Magnetic resonance imaging validation of pituitary gland compression and distortion by typical sellar pathology. J Neurosurg. 119:1461–1466. 2013.
Article11. George E, Heier L, Kovanlikaya I, Greenfield J. Diffusion tensor imaging of pyramidal tract reorganization after pediatric stroke. Childs Nerv Syst. 30:1135–1139. 2014.
Article12. Guo F, Song L, Bai J, Zhao P, Sun H, Liu X, et al. Successful treatment for giant pituitary adenomas through diverse transcranial approaches in a series of 15 consecutive patients. Clin Neurol Neurosurg. 114:885–890. 2012.
Article13. Harris GW, Manabe Y, Ruf KB. A study of the parameters of electrical stimulation of unmyelinated fibres in the pituitary stalk. J Physiol. 203:67–81. 1969.
Article14. Hasan KM, Kamali A, Kramer LA. Mapping the human brain white matter tracts relative to cortical and deep gray matter using diffusion tensor imaging at high spatial resolution. Magn Reson Imaging. 27:631–636. 2009.
Article15. Kamali A, Hasan KM, Adapa P, Razmandi A, Keser Z, Lincoln J, et al. Distinguishing and quantification of the human visual pathways using high spatial resolution diffusion tensor tractography. Magn Reson Imaging. 32:796–803. 2014.
Article16. Kovacs K, Horvath E. Pathology of pituitary tumors. Endocrinol Metab Clin North Am. 16:529–551. 1987.
Article17. Li HY, Feng CY, Zhang C, Su J, Yuan J, Xie Y, et al. Microscopic surgery for pituitary adenomas to preserve the pituitary gland and stalk. Exp Ther Med. 13:1011–1016. 2017.
Article18. Loyo-Varela M, Herrada-Pineda T, Revilla-Pacheco F, Manrique-Guzman S. Pituitary tumor surgery: review of 3004 cases. World Neurosurg. 79:331–336. 2013.
Article19. Ontaneda D, Sakaie K, Lin J, Wang X, Lowe MJ, Phillips MD, et al. Identifying the start of multiple sclerosis injury: a serial DTI study. J Neuroimaging. 24:569–576. 2014.
Article20. Pratheesh R, Rajaratnam S, Prabhu K, Mani SE, Chacko G, Chacko AG. The current role of transcranial surgery in the management of pituitary adenomas. Pituitary. 16:419–434. 2013.
Article21. Prieto R, Pascual JM, Rosdolsky M, Castro-Dufourny I, Carrasco R, Strauss S, et al. Craniopharyngioma adherence: a comprehensive topographical categorization and outcome-related risk stratification model based on the methodical examination of 500 tumors. Neurosurg Focus. 41:E13. 2016.
Article22. Psomiades M, Fonteneau C, Mondino M, Luck D, Haesebaert F, SuaudChagny MF, et al. Integrity of the arcuate fasciculus in patients with schizophrenia with auditory verbal hallucinations: a DTI-tractography study. Neuroimage Clin. 12:970–975. 2016.
Article23. Salmela MB, Cauley KA, Nickerson JP, Koski CJ, Filippi CG. Magnetic resonance diffusion tensor imaging (MRDTI) and tractography in children with septo-optic dysplasia. Pediatr Radiol. 40:708–713. 2010.
Article24. Satogami N, Miki Y, Koyama T, Kataoka M, Togashi K. Togashi normal pituitary stalk: high-resolution MR imaging at 3T. AJNR Am J Neuroradiol. 31:355–359. 2010.
Article25. Scheithauer BW. Surgical pathology of the pituitary: the adenomas. Part I. Pathol Annu 19 Pt. 1:317–374. 1984.26. Simmons GE, Suchnicki JE, Rak KM, Damiano TR. MR imaging of the pituitary stalk: size, shape, and enhancement pattern. AJR Am J Roentgenol. 159:375–377. 1992.
Article27. Stone BS, Zhang J, Mack DW, Mori S, Martin LJ, Northington FJ. Delayed neural network degeneration after neonatal hypoxia-ischemia. Ann Neurol. 64:535–546. 2008.
Article28. Sui M, Liu S, Liu M, Li Y, Tian Y. Locating of the pituitary stalk for craniopharyngioma surgery of transfrontobasal interhemispheric approach. J Craniofac Surg. 24:2106–2109. 2013.
Article29. Wang F, Zhang J, Wang P, Zhou T, Meng X, Jiang J. Prediction of pituitary stalk position in pituitary adenomas by visualization of the hypothalamo-hypophyseal tract using diffusion tensor imaging tractography. Medicine (Baltimore). 97:e0052. 2018.
Article30. Xiao G, Yuan X, Yuan J, Krumtally NA, Li Y, Feng C, et al. Pituitary stalk management during the microsurgery of craniopharyngiomas. Exp Ther Med. 7:1055–1064. 2014.
Article31. Yao Y, Ulrich NH, Guggenberger R, Alzarhani YA, Bertalanffy H, Kollias SS. Quantification of corticospinal tracts with diffusion tensor imaging in brainstem surgery: prognostic value in 14 consecutive cases at 3T magnetic resonance imaging. World Neurosurg. 83:1006–1014. 2015.
Article32. Zheng Z, Shemmassian S, Wijekoon C, Kim W, Bookheimer SY, Pouratian N. DTI correlates of distinct cognitive impairments in Parkinson’s disease. Hum Brain Mapp. 35:1325–1333. 2014.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Surgical Treatment of Hemangioblastoma in the Pituitary Stalk: An Extremely Rare Case
- Sporadic Hemangioblastoma in the Pituitary Stalk: A Case Report and Review of the Literature
- Comparative evaluation of CT and secreting hormones in pituitary adenomas
- Pituitary Stalk Transection Syndrome
- Characterization of the Anatomic Location of the Pituitary Stalk and Its Relationship to the Dorsum Sellae, Tuberculum Sellae and Chiasmatic Cistern