J Korean Neurosurg Soc.
2020 Jul;63(4):463-469. 10.3340/jkns.2019.0179.
Expression and Significance of MicroRNA155 in Serum of Patients with Cerebral Small Vessel Disease
- Affiliations
-
- 1Department of Neurology, Pu’er People’s Hospital, Pu’er, China
- 2Department of Internal Medicine, Pu’er City Prison Hospital, Pu’er, China
- KMID: 2504640
- DOI: http://doi.org/10.3340/jkns.2019.0179
Abstract
Objective
: This study aimed to investigate the changes and significance of microRNA155 levels in serum of patients with cerebral small vessel disease (CSVD).
Methods
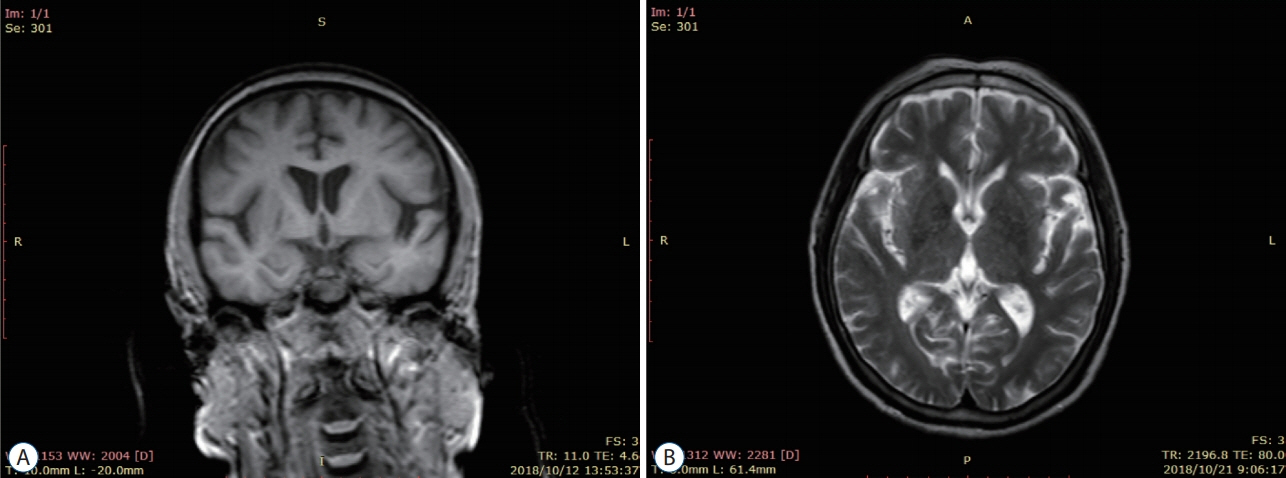
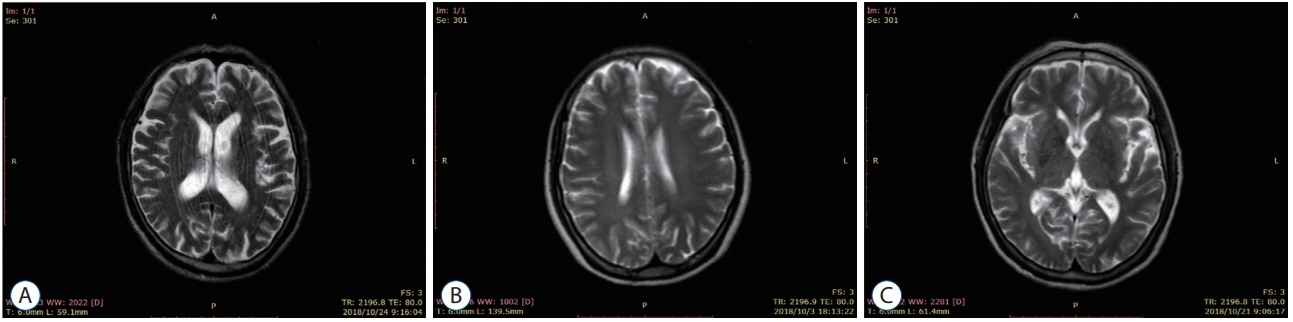
: Thirty patients with CSVD who met the inclusion criteria were selected and divided into eight patients with lacunar infarction (LI) group and 22 patients with multiple lacunar infarction (MLI) combined with white matter lesions (WML) group according to the results of head magnetic resonance imaging (MRI). Thirty samples from healthy volunteers without abnormalities after head MRI examination were selected as the control group. The levels of serum microRNA155 in each group were determined by real-time polymerase chain reaction, and the correlation between microRNA155 in the serum of patients with CSVD and the increase of imaging lesions was analyzed by Spearman correlation analysis.
Results
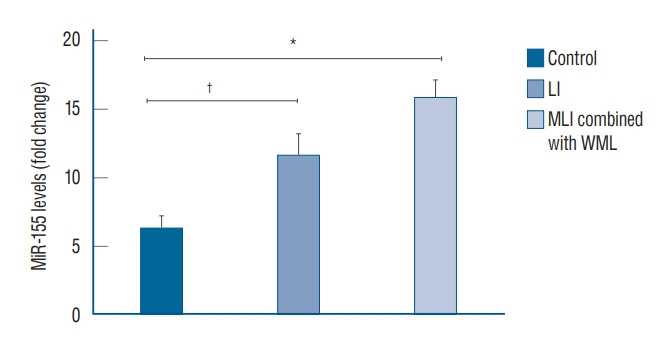
: Compared with the control group, the serum microRNA155 level in the LI group, MLI combined with WML group increased, the difference was statistically significant (p<0.05); serum microRNA155 level was positively correlated with the increase of imaging lesions (p<0.05).
Conclusion
: The change of serum microRNA155 level in patients with CSVD may be one of its self-protection mechanisms, and the intensity of this self-protection mechanism is positively correlated with the number of CSVD lesions.
Keyword
Figure
Reference
-
References
1. Ago T. The neurovascular unit in health and ischemic stroke. Nihon Rinsho. 74:583–588. 2016.2. Arai K, Lok J, Guo S, Hayakawa K, Xing C, Lo EH. Cellular mechanisms of neurovascular damage and repair after stroke. J Child Neurol. 26:1193–1198. 2011.
Article3. Cerebrovascular disease group of Neurology branch of Chinese Medical Association; Neurology Branch of Chinese Medical Association. Consensus on diagnosis and treatment of cerebrovascular disease in China. Chin J Neurol. 48:838–844. 2015.4. Chiu AP, Wan A, Lal N, Zhang D, Wang F, Vlodavsky I, et al. Cardiomyocyte VEGF regulates endothelial cell GPIHBP1 to relocate lipoprotein lipase to the coronary lumen during diabetes mellitus. Arterioscler Thromb Vasc Biol. 36:145–155. 2016.
Article5. Cuadrado-Godia E, Dwivedi P, Sharma S, Ois Santiago A, Roquer Gonzalez J, Balcells M, et al. Cerebral small vessel disease: a review focusing on pathophysiology, biomarkers, and machine learning strategies. J Stroke. 20:302–320. 2018.
Article6. Duan CL, Liu CW, Shen SW, Yu Z, Mo JL, Chen XH, et al. Striatal astrocytes transdifferentiate into functional mature neurons following ischemic brain injury. Glia. 63:1660–1670. 2015.
Article7. Garzon R, Croce CM. MicroRNAs in normal and malignant hematopoiesis. Curr Opin Hematol. 15:352–358. 2008.
Article8. Janaszak-Jasiecka A, Siekierzycka A, Bartoszewska S, Serocki M, Dobrucki LW, Collawn JF, et al. eNOS expression and NO release during hypoxia is inhibited by miR-200b in human endothelial cells. Angiogenesis. 21:711–724. 2018.
Article9. Kawasaki Y, Fujiki M, Uchida S, Morishige M, Momii Y, Ishii K. A single oral dose of geranylgeranylacetone upregulates vascular endothelial growth factor and protects against Kainic acid-induced neuronal cell death: involvement of the phosphatidylinositol-3 kinase/Akt pathway. Pathobiology. 84:184–191. 2017.
Article10. Latzer P, Schlegel U, Theiss C. Morphological changes of cortical and hippocampal neurons after treatment with VEGF and bevacizumab. CNS Neurosci Ther. 22:440–450. 2016.
Article11. Leng RX, Pan HF, Qin WZ, Chen GM, Ye DQ. Role of microRNA-155 in autoimmunity. Cytokine Growth Factor Rev. 22:141–147. 2011.
Article12. Lopez-Ramirez MA, Wu D, Pryce G, Simpson JE, Reijerkerk A, KingRobson J, et al. MicroRNA-155 negatively affects blood-brain barrier function during neuroinflammation. FASEB J. 28:2551–2565. 2014.
Article13. Markus HS, Hunt B, Palmer K, Enzinger C, Schmidt H, Schmidt R. Markers of endothelial and hemostatic activation and progression of cerebral white matter hyperintensities: longitudinal results of the Austrian Stroke Prevention Study. Stroke. 36:1410–1414. 2005.
Article14. McConnell HL, Kersch CN, Woltjer RL, Neuwelt EA. The translational significance of the neurovascular unit. J Biol Chem. 292:762–770. 2017.
Article15. Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 147:728–741. 2011.
Article16. Pan ZG, Mao Y, Sun FY. VEGF enhances reconstruction of neurovascular units in the brain after injury. Sheng Li Xue Bao. 69:96–108. 2017.17. Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 9:689–701. 2010.
Article18. Poggesi A, Pasi M, Pescini F, Pantoni L, Inzitari D. Circulating biologic markers of endothelial dysfunction in cerebral small vessel disease: a review. J Cereb Blood Flow Metab. 36:72–94. 2016.
Article19. Shen SW, Duan CL, Chen XH, Wang YQ, Sun X, Zhang QW, et al. Neurogenic effect of VEGF is related to increase of astrocytes transdifferentiation into new mature neurons in rat brains after stroke. Neuropharmacology. 108:451–461. 2016.
Article20. Silva-Hucha S, Hernández RG, Benítez-Temiño B, Pastor ÁM, de la Cruz RR, Morcuende S. Extraocular motoneurons of the adult rat show higher levels of vascular endothelial growth factor and its receptor Flk-1 than other cranial motoneurons. PLoS One. 12:e178616. 2017.
Article21. Sochor M, Basova P, Pesta M, Dusilkova N, Bartos J, Burda P, et al. Oncogenic microRNAs: miR-155, miR-19a, miR-181b, and miR-24 enable monitoring of early, breast cancer in serum. BMC Cancer. 14:448. 2014.
Article22. Staszel T, Zapala B, Polus A, Sadakierska-Chudy A, Kieć -Wilk B, Stępień E, et al. Role of microRNAs in endothelial cell pathophysiology. Pol Arch Med Wewn. 121:361–366. 2011.
Article23. Tang MM, Lin WJ, Pan YQ, Guan XT, Li YC. Hippocampal neurogenesis dysfunction linked to depressive-like behaviors in a neuroinflammation induced model of depression. Physiol Behav. 161:166–173. 2016.
Article24. Teng G, Papavasiliou FN. Shhh! Silencing by microRNA-155. Philos Trans R Soc Lond B Biol Sci. 364:631–637. 2009.
Article25. Wardlaw JM, Sandercock PA, Dennis MS, Starr J. Is breakdown of the blood-brain barrier responsible for lacunar stroke, leukoaraiosis, and dementia? Stroke. 34:806–812. 2003.
Article26. Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol. 12:483–497. 2013.
Article27. Wu KW, Yang P, Li SS, Liu CW, Sun FY. VEGF attenuated increase of outward delayed-rectifier potassium currents in hippocampal neurons induced by focal ischemia via PI3-K pathway. Neuroscience. 298:94–101. 2015.
Article28. Wu Q, Jin H, Yang Z, Luo G, Lu Y, Li K, et al. MiR-150 promotes gastric cancer proliferation by negatively regulating the pro-apoptotic gene EGR2. Biochem Biophys Res Commun. 392:340–345. 2010.
Article29. Xie XG, Zhang M, Dai YK, Ding MS, Meng SD. Combination of vascular endothelial growth factor-loaded microspheres and hyperbaric oxygen on random skin flap survival in rats. Exp Ther Med. 10:954–958. 2015.
Article30. Yang J, Yang C, Liu C, Zhang T, Yang Z. Paradoxical effects of VEGF on synaptic activity partially involved in notch1 signaling in the mouse hippocampus. Hippocampus. 26:589–600. 2016.
Article31. Yassi N, Desmond PM, Masters CL. Magnetic resonance imaging of vascular contributions to cognitive impairment and dementia. J Mol Neurosci. 60:349–353. 2016.
Article32. Yin KJ, Deng Z, Hamblin M, Xiang Y, Huang H, Zhang J, et al. Peroxisome proliferator-activated receptor delta regulation of miR-15a in ischemia-induced cerebral vascular endothelial injury. J Neurosci. 30:6398–6408. 2010.
Article33. Zhang CM, Zhao J, Deng HY. MiR-155 promotes proliferation of human breast cancer MCF-7 cells through targeting tumor protein 53-induced nuclear protein 1. J Biomed Sci. 20:79. 2013.
Article34. Zhang J, Zou H, Zhang Q, Wang L, Lei J, Wang Y, et al. Effects of Xiaoshuan enteric-coated capsule on neurovascular functions assessed by quantitative multiparametric MRI in a rat model of permanent cerebral ischemia. BMC Complement Altern Med. 16:198. 2016.
Article35. Zhang L, Wang W, Li X, He S, Yao J, Wang X, et al. MicroRNA-155 promotes tumor growth of human hepatocellular carcinoma by targeting ARID2. Int J Oncol. 48:2425–2434. 2016.
Article36. Zhou ZC, Gu SZ, Wu J, Liang QW. VEGF, eNOS, and ABCB1 genetic polymorphisms may increase the risk of osteonecrosis of the femoral head. Genet Mol Res. 14:13688–13698. 2015.
Article37. Zhu X, Wang Y, Sun Y, Zheng J, Zhu D. MiR-155 up-regulation by LMP1 DNA contributes to increased nasopharyngeal carcinoma cell proliferation and migration. Eur Arch Otorhinolaryngol. 271:1939–1945. 2014.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Vascular Overload Index Predicts Cerebral Small Vessel Disease in a Neurologically Healthy Population
- Relationship between Methylenetetrahydrofolate Reductase C677T Homozygous Mutation and Cerebral Small Vessel Disease Subtypes
- Cerebral Small Vessel Disease and Chronic Kidney Disease
- Evolving Concept of Small Vessel Disease through Advanced Brain Imaging
- Association between Cerebral Small Vessel and Alzheimer’s Disease