J Korean Neurosurg Soc.
2020 Jul;63(4):444-454. 10.3340/jkns.2019.0252.
The Dose Dependent Effects of Ruxolitinib on the Invasion and Tumorigenesis in Gliomas Cells via Inhibition of Interferon Gamma-Depended JAK/STAT Signaling Pathway
- Affiliations
-
- 1Department of Neurosurgery, Trakya University School of Medicine, Edirne, Turkey
- 2Department of Medical Biology, Trakya University School of Medicine, Edirne, Turkey
- KMID: 2504638
- DOI: http://doi.org/10.3340/jkns.2019.0252
Abstract
Objective
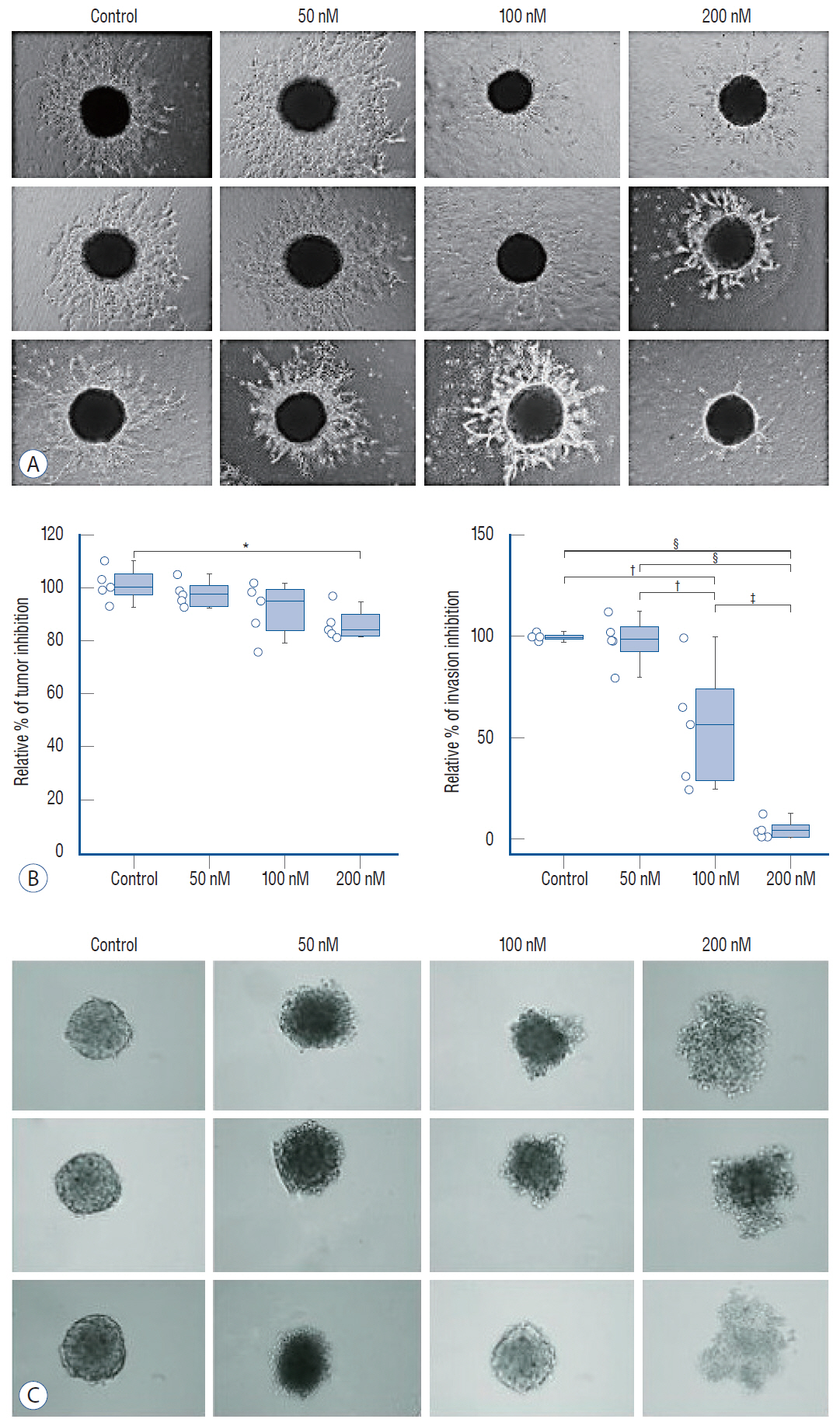
: Glioblastoma multiforme (GBM) is the most aggressive for of brain tumor and treatment often fails due to the invasion of tumor cells into neighboring healthy brain tissues. Activation of the Janus kinase-signal transducer and activator of transcription (JAK/STAT) signaling pathway is essential for normal cellular function including angiogenesis, and has been proposed to have a pivotal role in glioma invasion. This study aimed to determine the dose-dependent effects of ruxolitinib, an inhibitor of JAK, on the interferon (IFN)-I/IFN-α/IFN-β receptor/STAT and IFN-γ/IFN-γ receptor/STAT1 axes of the IFN-receptor-dependent JAK/STAT signaling pathway in glioblastoma invasion and tumorigenesis in U87 glioblastoma tumor spheroids.
Methods
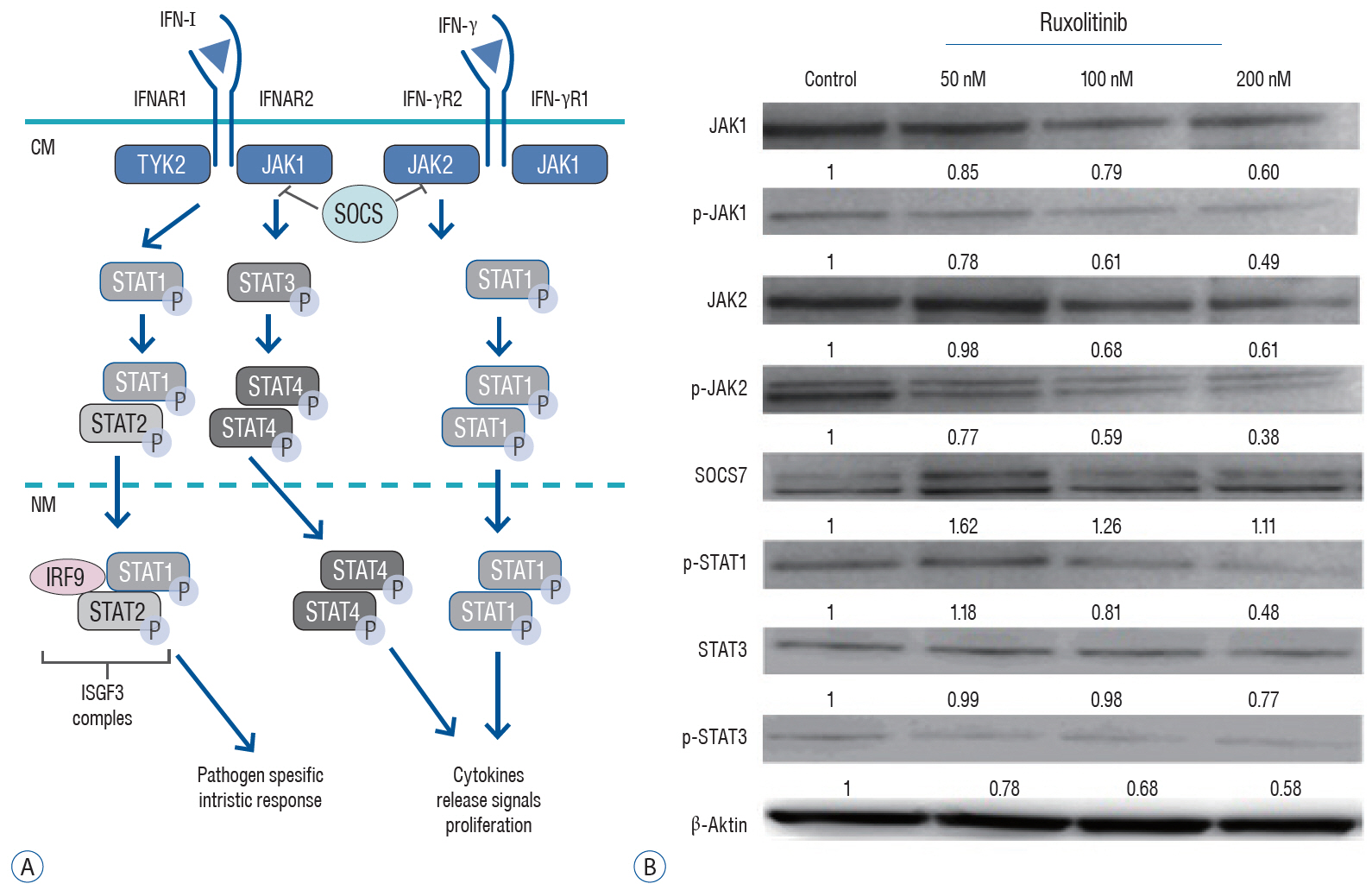
: We administered three different doses of ruxolitinib (50, 100, and 200 nM) to human U87 glioblastoma spheroids and analyzed the gene expression profiles of IFNs receptors from the JAK/STAT pathway. To evaluate activation of this pathway, we quantified the phosphorylation of JAK and STAT proteins using Western blotting.
Results
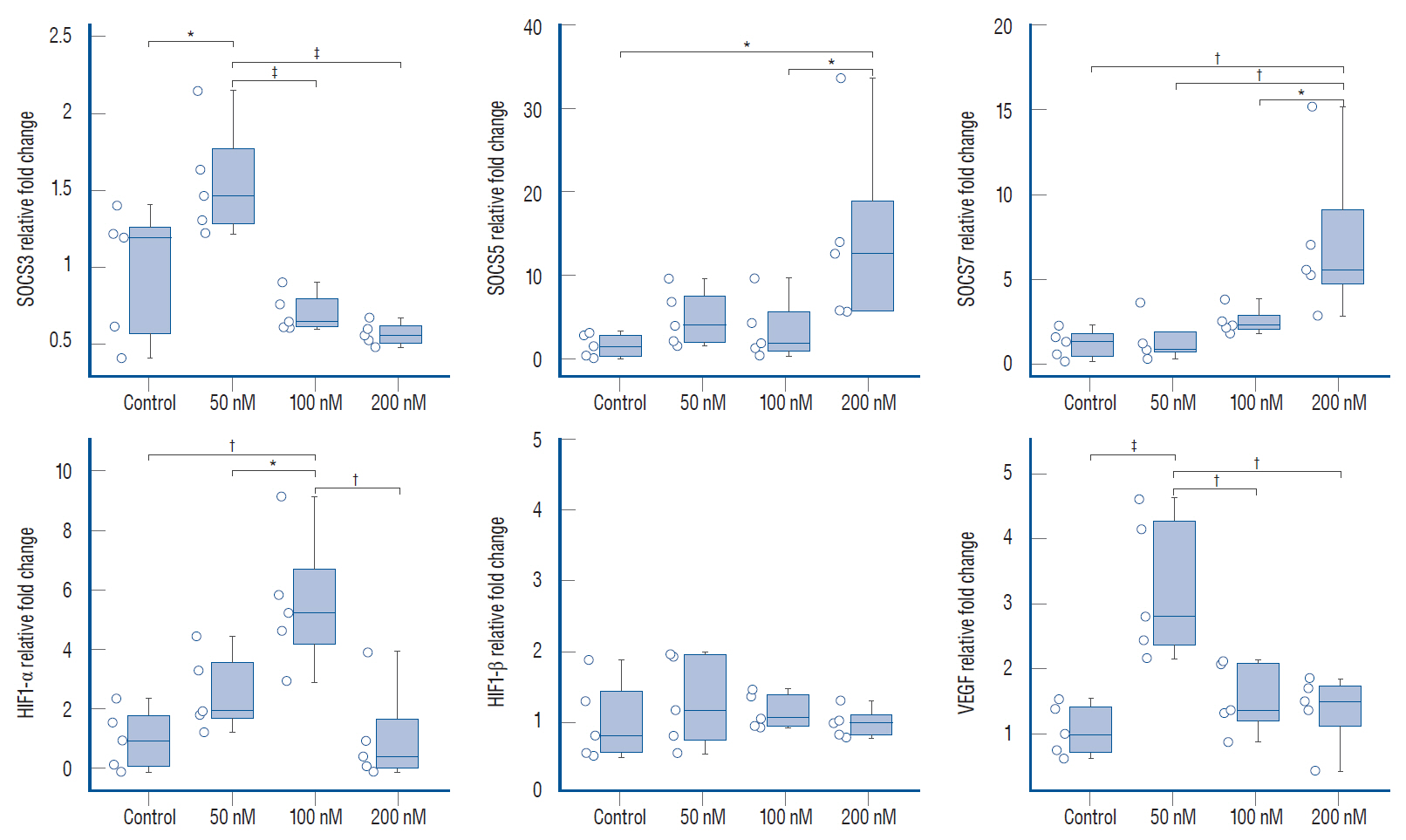
: Quantitative real-time polymerase chain reaction analysis demonstrated that ruxolitinib led to upregulated of the IFN-α and IFN-γ while no change on the hypoxia-inducible factor-1α and vascular endothelial growth factor expression levels. Additionally, we showed that ruxolitinib inhibited phosphorylation of JAK/STAT proteins. The inhibition of IFNs dependent JAK/STAT signaling by ruxolitinib leads to decreases of the U87 cells invasiveness and tumorigenesis. We demonstrate that ruxolitinib may inhibit glioma invasion and tumorigenesis through inhibition of the IFN-induced JAK/STAT signaling pathway.
Conclusion
: Collectively, our results revealed that ruxolitinib may have therapeutic potential in glioblastomas, possibly by JAK/STAT signaling triggered by IFN-α and IFN-γ.
Figure
Reference
-
References
1. Atkinson GP, Nozell SE, Benveniste ETN. NF-kappaB and STAT3 signaling in glioma: targets for future therapies. Expert Rev Neurother. 10:575–586. 2010.2. Batash R, Asna N, Schaffer P, Francis N, Schaffer M. Glioblastoma multiforme, diagnosis and treatment; recent literature review. Curr Med Chem. 24:3002–3009. 2017.
Article3. Charras A, Arvaniti P, Le Dantec C, Arleevskaya MI, Zachou K, Dalekos GN, et al. JAK inhibitors suppress innate epigenetic reprogramming: a promise for patients with Sjögren’s syndrome. Clin Rev Allergy Immunol. 58:182–193. 2020.
Article4. Cheng Z, Fu J, Liu G, Zhang L, Xu Q, Wang SY. Angiogenesis in JAK2 V617F positive myeloproliferative neoplasms and ruxolitinib decrease VEGF, HIF-1 enesis in JAK2 V617F positive cells. Leuk Lymphoma. 59:196–203. 2018.
Article5. Clara CA, Marie SK, de Almeida JR, Wakamatsu A, Oba-Shinjo SM, Uno M, et al. Angiogenesis and expression of PDGF-C, VEGF, CD105 and HIF-1α in human glioblastoma. Neuropathology. 34:343–352. 2014.
Article6. Delen E, Doganlar O, Doganlar ZB, Delen O. Inhibition of the invasion of human glioblastoma U87 cell line by ruxolitinib: a molecular player of miR-17 and miR-20a regulating JAK/STAT pathway. Turk Neurosurg. 30:182–189. 2020.
Article7. Del Duca D, Werbowetski T, Del Maestro RF. Spheroid preparation from hanging drops: characterization of a model of brain tumor invasion. J Neurooncol. 67:295–303. 2004.
Article8. Fetell MR, Housepian EM, Oster MW, Cote DN, Sisti MB, Marcus SG, et al. Intratumor administration of beta-interferon in recurrent malignant gliomas. A phase I clinical and laboratory study. Cancer. 65:78–83. 1990.
Article9. Fogelman D, Cubillo A, García-Alfonso P, Mirón MLL, Nemunaitis J, Flora D, et al. Randomized, double-blind, phase two study of ruxolitinib plus regorafenib in patients with relapsed/refractory metastatic colorectal cancer. Cancer Med. 7:5382–5393. 2018.
Article10. George J, Banik NL, Ray SK. Knockdown of hTERT and concurrent treatment with interferon-gamma inhibited proliferation and invasion of human glioblastoma cell lines. Int J Biochem Cell Biol. 42:1164–1173. 2010.
Article11. Haile WB, Gavegnano C, Tao S, Jiang Y, Schinazi RF, Tyor WR. The Janus kinase inhibitor ruxolitinib reduces HIV replication in human macrophages and ameliorates HIV encephalitis in a murine model. Neurobiol Dis. 92(Pt B):137–143. 2016.
Article12. Han ES, Wen W, Dellinger TH, Wu J, Lu SA, Jove R, et al. Ruxolitinib synergistically enhances the anti-tumor activity of paclitaxel in human ovarian cancer. Oncotarget. 9:24304–24319. 2018.
Article13. Honda S, Sadatomi D, Yamamura Y, Nakashioya K, Tanimura S, Takeda K. WP1066 suppresses macrophage cell death induced by inflammasome agonists independently of its inhibitory effect on STAT3. Cancer Sci. 108:520–527. 2017.
Article14. Hurwitz HI, Uppal N, Wagner SA, Bendell JC, Beck JT, Wade SM 3rd, et al. Randomized, double-blind, phase II study of ruxolitinib or placebo in combination with capecitabine in patients with metastatic pancreatic cancer for whom therapy with gemcitabine has failed. J Clin Oncol. 33:4039–4047. 2015.
Article15. Jain RK, di Tomaso E, Duda DG, Loeffler JS, Sorensen AG, Batchelor TT. Angiogenesis in brain tumours. Nat Rev Neurosci. 8:610–622. 2007.
Article16. Jensen KV, Cseh O, Aman A, Weiss S, Luchman HA. The JAK2/STAT3 inhibitor pacritinib effectively inhibits patient-derived GBM brain tumor initiating cells in vitro and when used in combination with temozolomide increases survival in an orthotopic xenograft model. PLoS One. 12:e0189670. 2017.
Article17. Kaur B, Khwaja FW, Severson EA, Matheny SL, Brat DJ, Van Meir EG. Hypoxia and the hypoxia-inducible-factor pathway in glioma growth and angiogenesis. Neuro Oncol. 7:134–153. 2005.
Article18. Kim EK, Chung DS, Shin HJ, Hong YK. Inhibitory effect of IFN-beta, on the Antitumor activity of celecoxib in U87 glioma model. J Korean Neurosurg Soc. 46:552–557. 2009.
Article19. Knüpfer MM, Knüpfer H, Van Gool S, Domula M, Wolff JE. Interferon gamma inhibits proliferation and hyaluronic acid adhesion of human malignant glioma cells in vitro. Cytokine. 12:409–412. 2000.
Article20. Li H, Liang Q, Wang L. Icaritin inhibits glioblastoma cell viability and glycolysis by blocking the IL-6/Stat3 pathway. J Cell Biochem. 120:7257–7264. 2019.
Article21. Loboda A, Jozkowicz A, Dulak J. HIF-1 and HIF-2 transcription factors--similar but not identical. Mol Cells. 29:435–442. 2010.
Article22. Mukthavaram R, Ouyang X, Saklecha R, Jiang P, Nomura N, Pingle SC, et al. Effect of the JAK2/STAT3 inhibitor SAR317461 on human glioblastoma tumorspheres. J Transl Med. 13:269. 2015.
Article23. Ohno M, Natsume A, Kondo Y, Iwamizu H, Motomura K, Toda H, et al. The modulation of microRNAs by type I IFN through the activation of signal transducers and activators of transcription 3 in human glioma. Mol Cancer Res. 7:2022–2030. 2009.
Article24. Price SJ, Gillard JH. Imaging biomarkers of brain tumour margin and tumour invasion. Br J Radiol 84 Spec No. 2:S159–S167. 2011.
Article25. Priyanka R, Muralidharan NP. Interferons and interferon therapy. J Pharm Sci Res. 6:400–403. 2014.26. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 9:671–675. 2012.
Article27. Silginer M, Nagy S, Happold C, Schneider H, Weller M, Roth P. Autocrine activation of the IFN signaling pathway may promote immune escape in glioblastoma. Neuro Oncol. 19:1338–1349. 2017.
Article28. Tate MC, Aghi MK. Biology of angiogenesis and invasion in glioma. Neurotherapeutics. 6:447–457. 2009.
Article29. Vallatos A, Al-Mubarak HFI, Birch JL, Galllagher L, Mullin JM, Gilmour L, et al. Quantitative histopathologic assessment of perfusion MRI as a marker of glioblastoma cell infiltration in and beyond the peritumoral edema region. J Magn Reson Imaging. 50:529–540. 2019.
Article30. Wakabayashi T, Kayama T, Nishikawa R, Takahashi H, Hashimoto N, Takahashi J, et al. A multicenter phase I trial of combination therapy with interferon-β and temozolomide for high-grade gliomas (INTEGRA study): the final report. J Neurooncol. 104:573–577. 2011.
Article31. Wei J, Ma L, Li C, Pierson CR, Finlay JL, Lin J. Targeting upstream kinases of STAT3 in human medulloblastoma cells. Curr Cancer Drug Targets. 19:571–582. 2019.
Article32. Weiswald LB, Bellet D, Dangles-Marie V. Spherical cancer models in tumor biology. Neoplasia. 17:1–15. 2015.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cytokine Signaling in Tumor Progression
- 15-Deoxy-delta12,14-PGJ2inhibits IL-6-induced Stat3 phosphorylation in lymphocytes
- Role of IFNLR1 gene in PRRSV infection of PAM cells
- Magnolol exerts anti-asthmatic effects by regulating Janus kinase-signal transduction and activation of transcription and Notch signaling pathways and modulating Th1/Th2/Th17 cytokines in ovalbumin-sensitized asthmatic mice
- Janus kinase inhibitors for the treatment of rheumatoid arthritis