Cancer Res Treat.
2020 Jul;52(3):957-972. 10.4143/crt.2019.695.
Computed Tomography–Determined Sarcopenia Is a Useful Imaging Biomarker for Predicting Postoperative Outcomes in Elderly Colorectal Cancer Patients
- Affiliations
-
- 1Deparment of Colorectal and Anal Surgery, The First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi, China
- 2Deparment of Pharmacy, The First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi, China
- KMID: 2504474
- DOI: http://doi.org/10.4143/crt.2019.695
Abstract
- Purpose
This study aimed to establish whether computed tomography (CT)–determined sarcopenia is a useful imaging biomarker for postoperative outcome in elderly colorectal cancer (CRC) patients, and construct sarcopenia-based nomograms to predict individual outcomes after surgery.
Materials and Methods
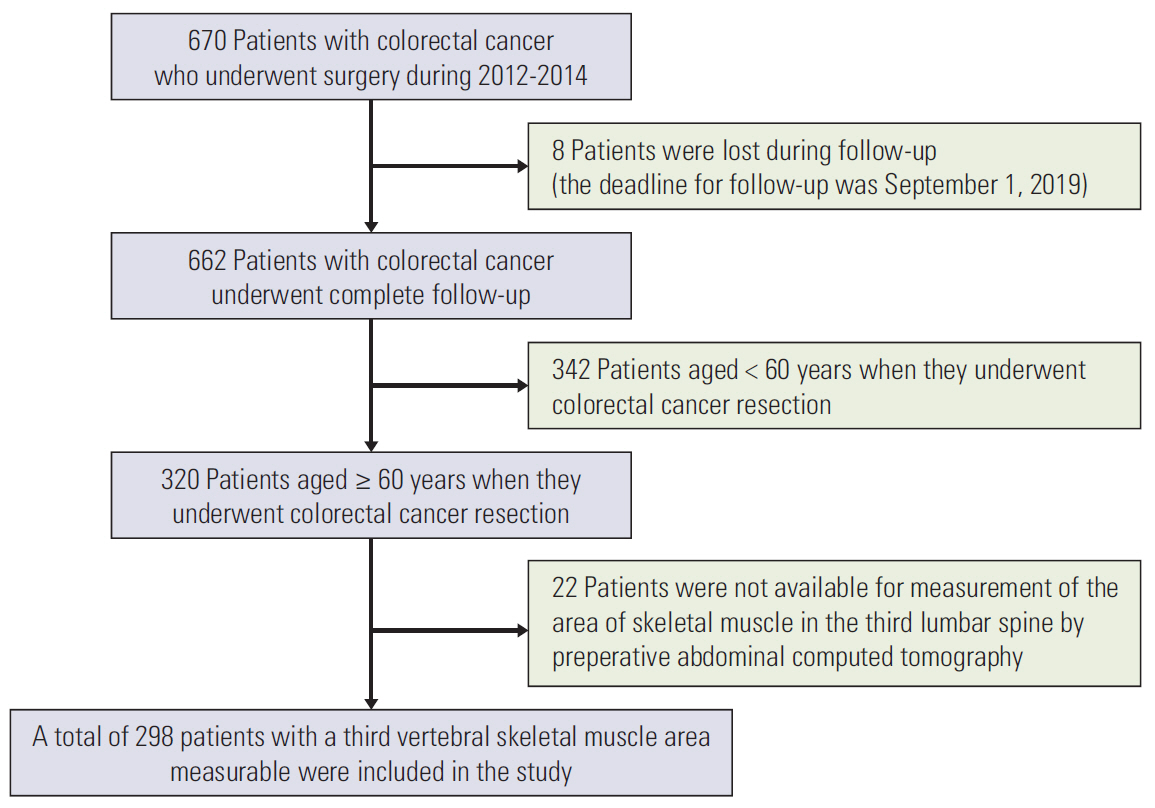
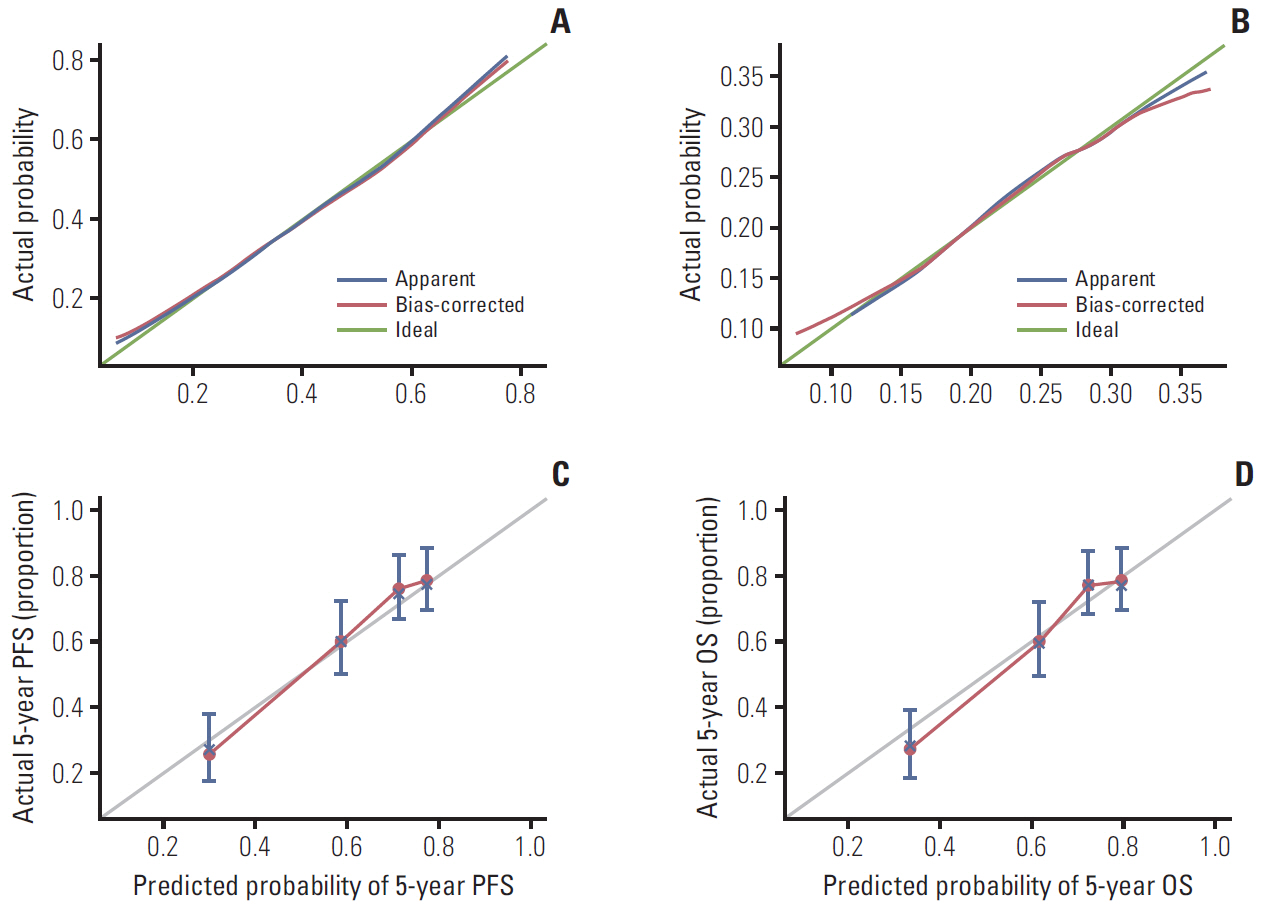
CT imaging data of 298 elderly CRC patients who underwent surgery in 2012-2014 were retrospectively analyzed. Skeletal muscle mass was determined by CT, and sarcopenia was diagnosed based on the optimal cutoff value determined by X-tile program. The correlation between sarcopenia and risk of preoperative nutrition and postoperative complications was evaluated. A Cox proportional hazards model was used to determine survival predictors. Sarcopenia-based nomograms were developed based on multivariate analysis, and calibrated using concordance index and calibration curves.
Results
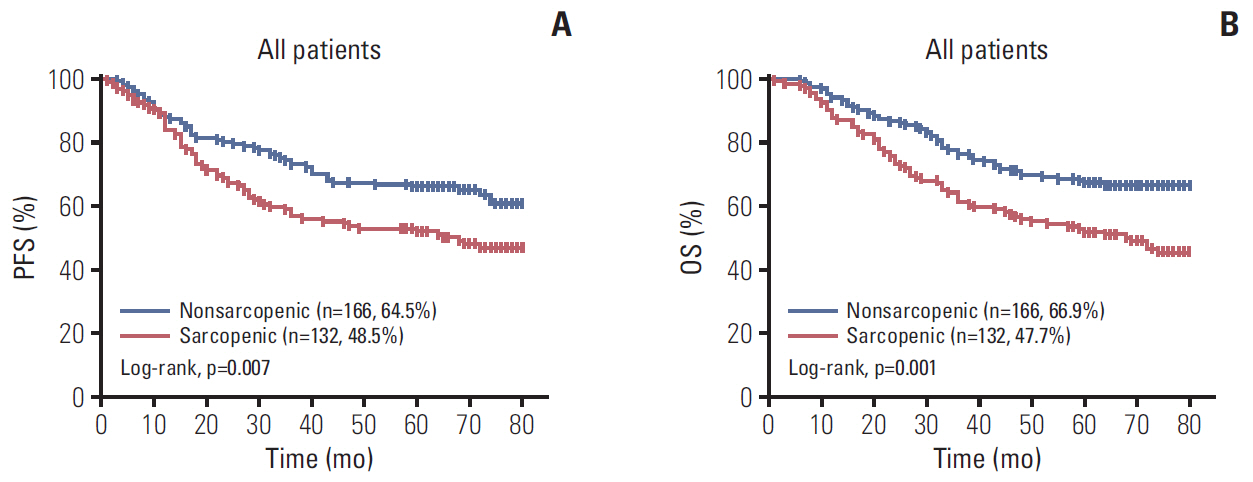
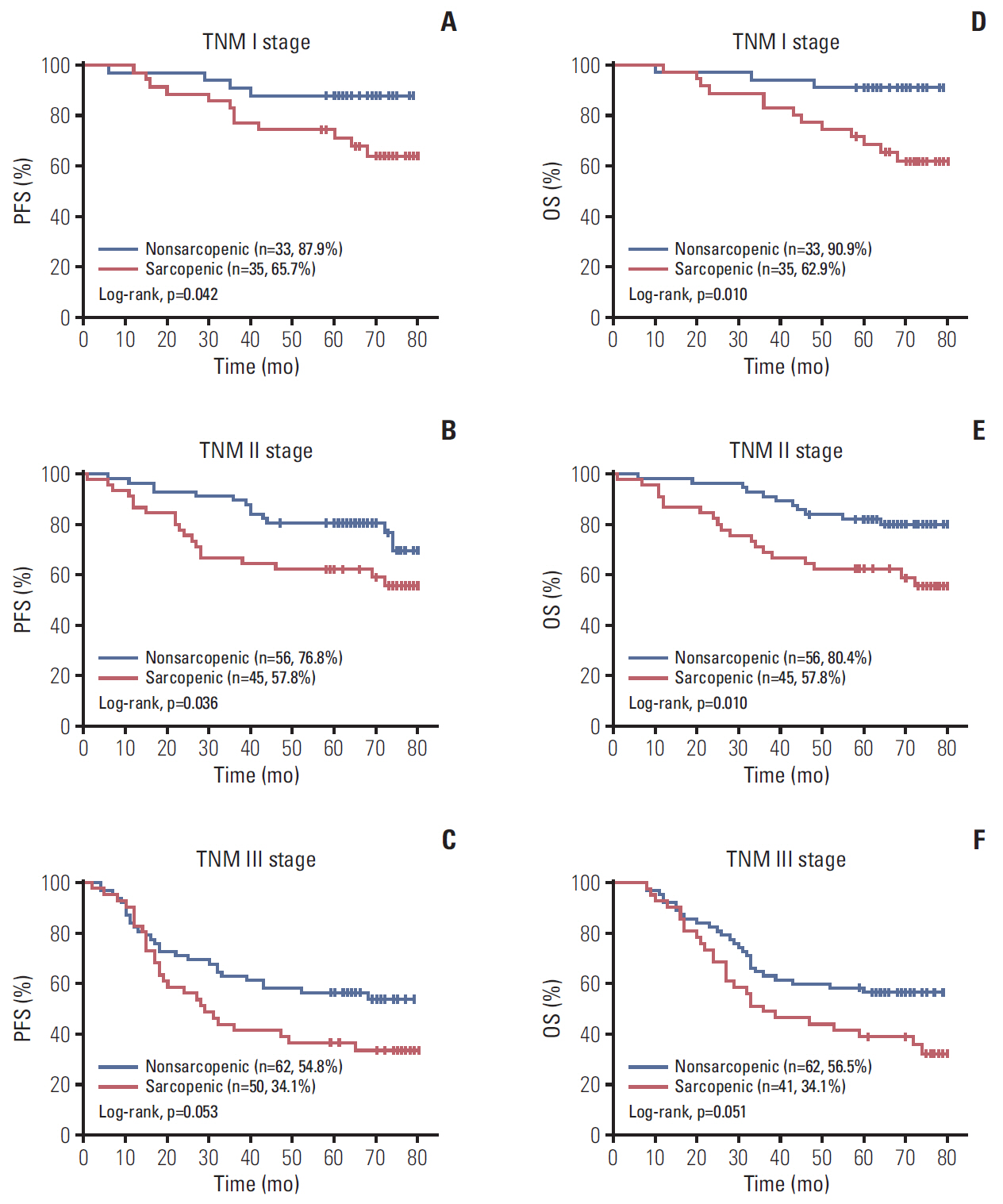
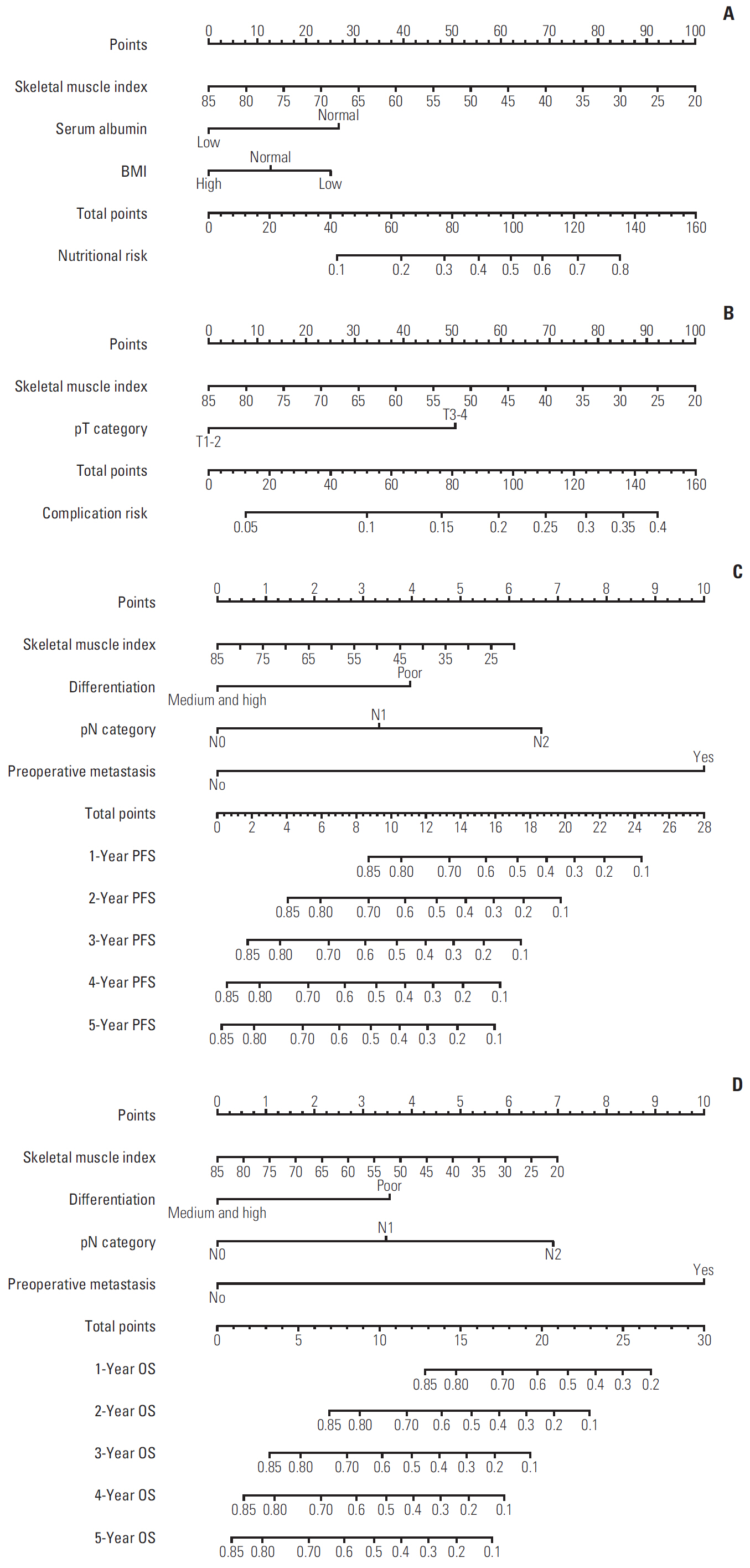
A total 132 patients (44.3%) had sarcopenia based on the optimum cutoff values (29.9 cm2/m2 for women and 49.5 cm2/m2 for men). Sarcopenia was an independent risk factor for preoperative nutrition (p < 0.001; odds ratio [OR], 3.405; 95% confidence interval [CI], 1.948 to 5.954) and postoperative complications (p=0.008; OR, 2.192; 95% CI, 1.231 to 3.903). Sarcopenia was an independent predictor for poor progression-free survival (p < 0.001; hazard ratio [HR], 2.175; 95% CI, 1.489 to 3.179) and overall survival (p < 0.001; HR, 2.524; 95% CI, 1.721 to 3.703). Based on multivariate analysis, we produced four nomograms that had better predictive performance.
Conclusion
CT-determined sarcopenia is a useful imaging biomarker for predicting preoperative nutritional risk, postoperative complications, and long-term outcomes in elderly CRC patients. The sarcopenia-based nomograms can provide a scientific basis for guiding therapeutic schedule and follow-up strategies.
Figure
Reference
-
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424.
Article2. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016; 66:115–32.
Article3. Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017; 66:683–91.
Article4. van Steenbergen LN, Elferink MA, Krijnen P, Lemmens VE, Siesling S, Rutten HJ, et al. Improved survival of colon cancer due to improved treatment and detection: a nationwide population-based study in The Netherlands 1989-2006. Ann Oncol. 2010; 21:2206–12.
Article5. Quaglia A, Tavilla A, Shack L, Brenner H, Janssen-Heijnen M, Allemani C, et al. The cancer survival gap between elderly and middle-aged patients in Europe is widening. Eur J Cancer. 2009; 45:1006–16.
Article6. Verweij NM, Schiphorst AH, Maas HA, Zimmerman DD, van den Bos F, Pronk A, et al. Colorectal Cancer Resections in the Oldest Old Between 2011 and 2012 in The Netherlands. Ann Surg Oncol. 2016; 23:1875–82.
Article7. Hamaker ME, Prins MC, Schiphorst AH, van Tuyl SA, Pronk A, van den Bos F. Long-term changes in physical capacity after colorectal cancer treatment. J Geriatr Oncol. 2015; 6:153–64.
Article8. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010; 39:412–23.
Article9. Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997; 127(5 Suppl):990S–1S.
Article10. Wallengren O, Iresjo BM, Lundholm K, Bosaeus I. Loss of muscle mass in the end of life in patients with advanced cancer. Support Care Cancer. 2015; 23:79–86.
Article11. Malietzis G, Currie AC, Johns N, Fearon KC, Darzi A, Kennedy RH, et al. Skeletal muscle changes after elective colorectal cancer resection: a longitudinal study. Ann Surg Oncol. 2016; 23:2539–47.
Article12. Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008; 33:997–1006.
Article13. Shachar SS, Williams GR, Muss HB, Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur J Cancer. 2016; 57:58–67.
Article14. Simonsen C, de Heer P, Bjerre ED, Suetta C, Hojman P, Pedersen BK, et al. Sarcopenia and postoperative complication risk in gastrointestinal surgical oncology: a meta-analysis. Ann Surg. 2018; 268:58–69.15. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004; 240:205–13.16. Lieffers JR, Bathe OF, Fassbender K, Winget M, Baracos VE. Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br J Cancer. 2012; 107:931–6.
Article17. Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bioinformatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004; 10:7252–9.18. Baracos VE. Regulation of skeletal-muscle-protein turnover in cancer-associated cachexia. Nutrition. 2000; 16:1015–8.
Article19. Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab. 2012; 16:153–66.
Article20. Rong YD, Bian AL, Hu HY, Ma Y, Zhou XZ. Study on relationship between elderly sarcopenia and inflammatory cytokine IL-6, anti-inflammatory cytokine IL-10. BMC Geriatr. 2018; 18:308.
Article21. Srikanthan P, Hevener AL, Karlamangla AS. Sarcopenia exacerbates obesity-associated insulin resistance and dysglycemia: findings from the National Health and Nutrition Examination Survey III. PLoS One. 2010; 5:e10805.
Article22. Tsoli M, Robertson G. Cancer cachexia: malignant inflammation, tumorkines, and metabolic mayhem. Trends Endocrinol Metab. 2013; 24:174–83.
Article23. van Vugt JL, Coebergh van den Braak RR, Lalmahomed ZS, Vrijland WW, Dekker JW, Zimmerman DD, et al. Impact of low skeletal muscle mass and density on short and long-term outcome after resection of stage I-III colorectal cancer. Eur J Surg Oncol. 2018; 44:1354–60.
Article24. Hall JC. Nutritional assessment of surgery patients. J Am Coll Surg. 2006; 202:837–43.
Article25. Prado CM, Heymsfield SB. Lean tissue imaging: a new era for nutritional assessment and intervention. JPEN J Parenter Enteral Nutr. 2014; 38:940–53.26. Black D, Mackay C, Ramsay G, Hamoodi Z, Nanthakumaran S, Park KG, et al. Prognostic value of computed tomography: measured parameters of body composition in primary operable gastrointestinal cancers. Ann Surg Oncol. 2017; 24:2241–51.
Article27. Sueda T, Takahasi H, Nishimura J, Hata T, Matsuda C, Mizushima T, et al. Impact of low muscularity and myosteatosis on long-term outcome after curative colorectal cancer surgery: a propensity score-matched analysis. Dis Colon Rectum. 2018; 61:364–74.
Article28. Malietzis G, Currie AC, Athanasiou T, Johns N, Anyamene N, Glynne-Jones R, et al. Influence of body composition profile on outcomes following colorectal cancer surgery. Br J Surg. 2016; 103:572–80.
Article29. Padilha CS, Marinello PC, Galvao DA, Newton RU, Borges FH, Frajacomo F, et al. Evaluation of resistance training to improve muscular strength and body composition in cancer patients undergoing neoadjuvant and adjuvant therapy: a meta-analysis. J Cancer Surviv. 2017; 11:339–49.
Article30. Ewaschuk JB, Almasud A, Mazurak VC. Role of n-3 fatty acids in muscle loss and myosteatosis. Appl Physiol Nutr Metab. 2014; 39:654–62.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A study on the comparision of various imaging methods for the staging of renal cell carcinoma
- Prevalence and associated risk of advanced colorectal neoplasia in adults with sarcopenia
- Clinical impact of sarcopenia in patients with colon cancer undergoing laparoscopic surgery
- Multimodal prerehabilitation for elderly patients with sarcopenia in colorectal surgery
- Accuracy of Preoperative Local Staging of Primary Colorectal Cancer by Using Computed Tomography: Reappraisal Based on Data Collected at a Highly Organized Cancer Center