Cancer Res Treat.
2020 Jul;52(3):945-956. 10.4143/crt.2020.080.
Inhibition of ATR Increases the Sensitivity to WEE1 Inhibitor in Biliary Tract Cancer
- Affiliations
-
- 1Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea
- 2Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea
- KMID: 2504473
- DOI: http://doi.org/10.4143/crt.2020.080
Abstract
- Purpose
Currently, the DNA damage response (DDR) pathway represents a key target for new cancer drug development. Advanced biliary tract cancer (BTC) has a poor prognosis because of the lack of efficacious treatment options. Although DNA repair pathway alterations have been reported in many patients with BTC, little is known regarding the effects of DDR-targeted agents against BTC.
Materials and Methods
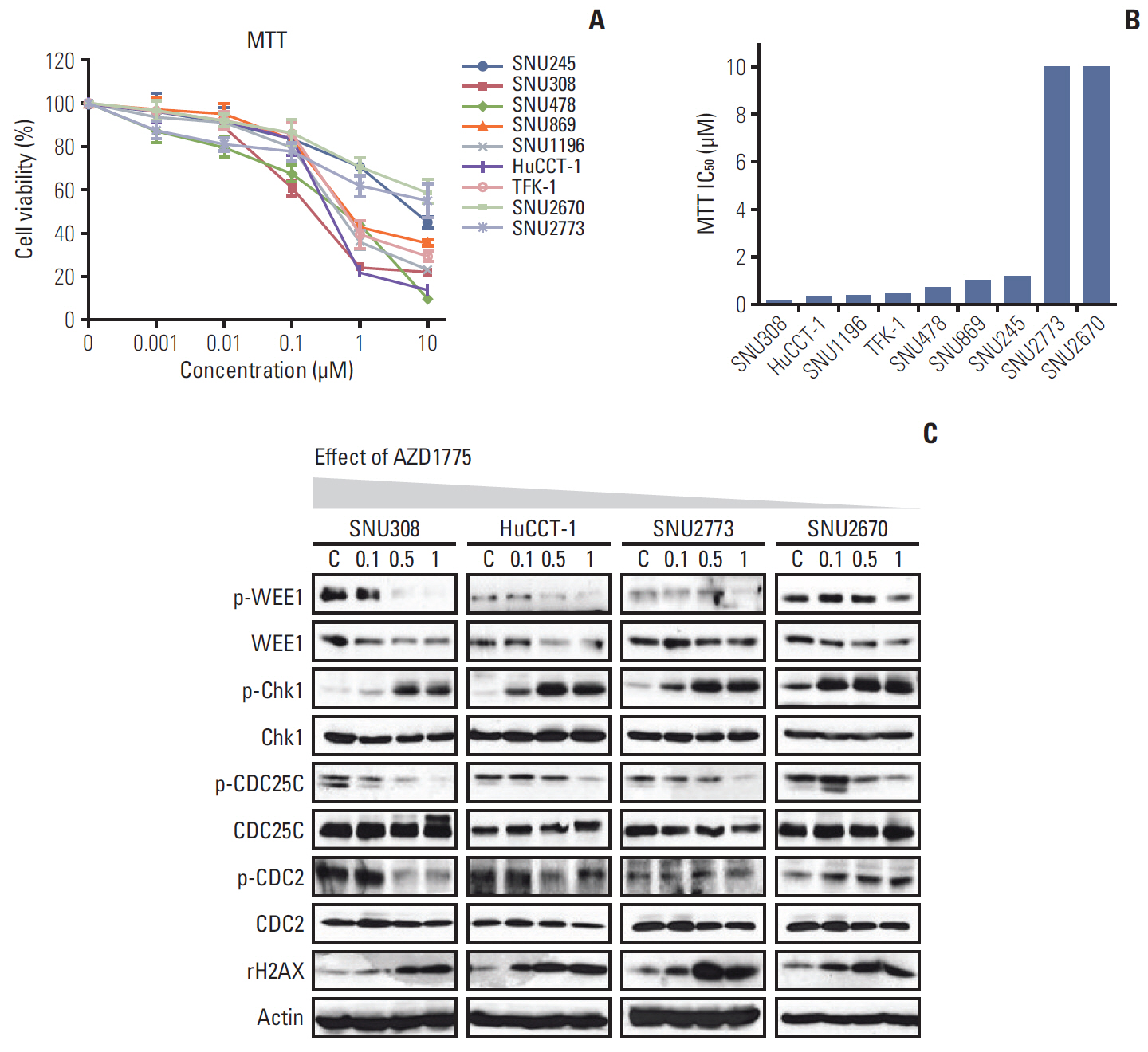
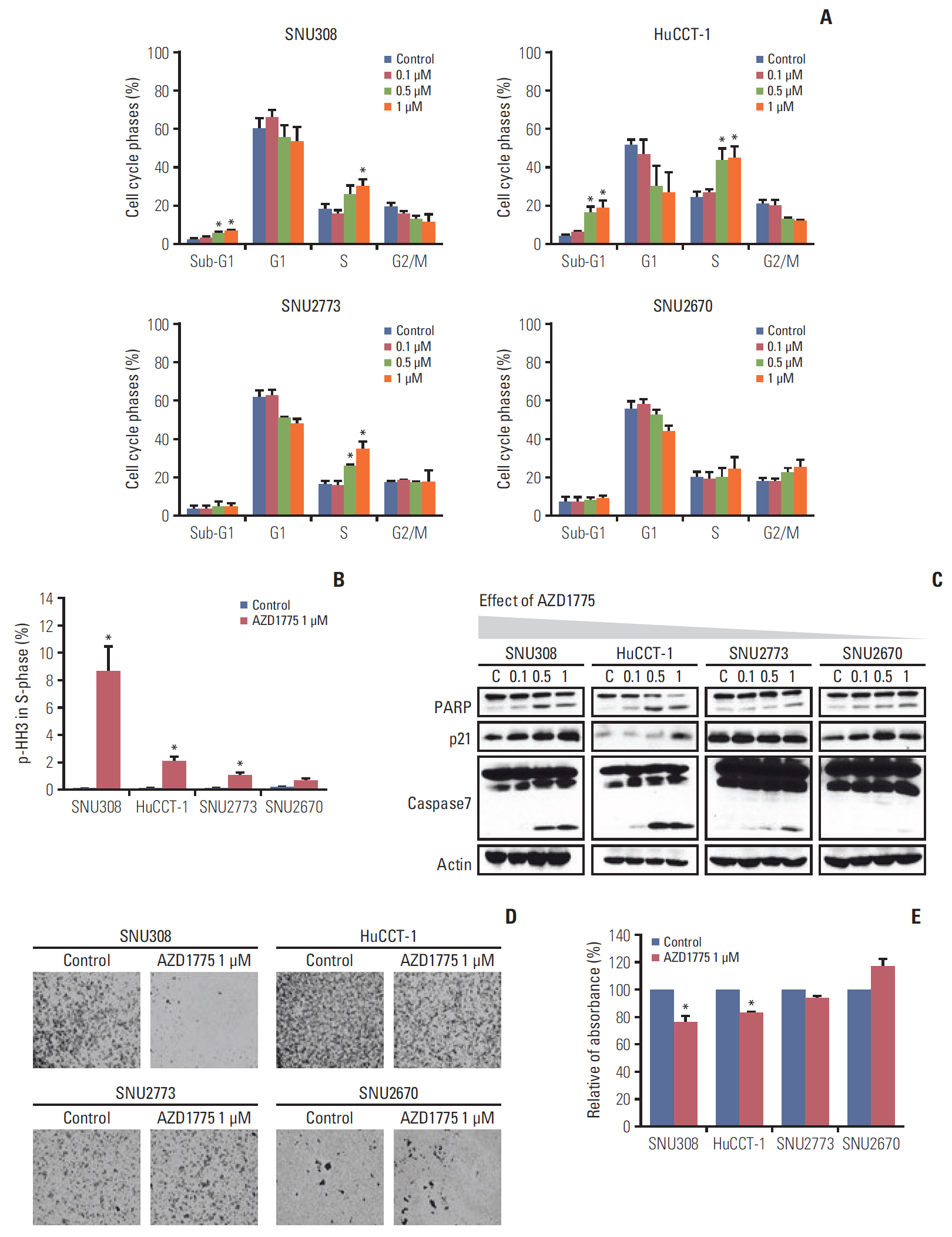
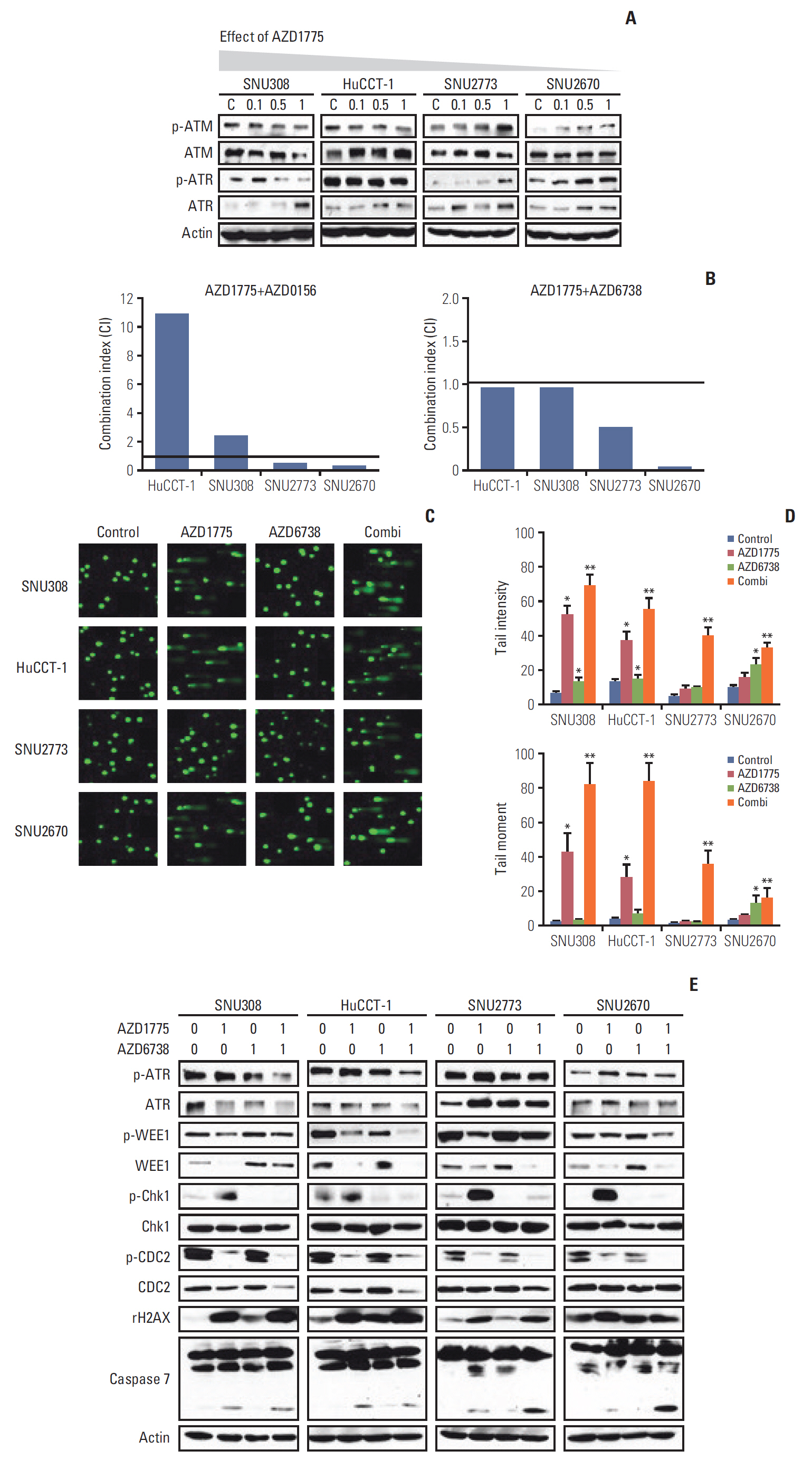
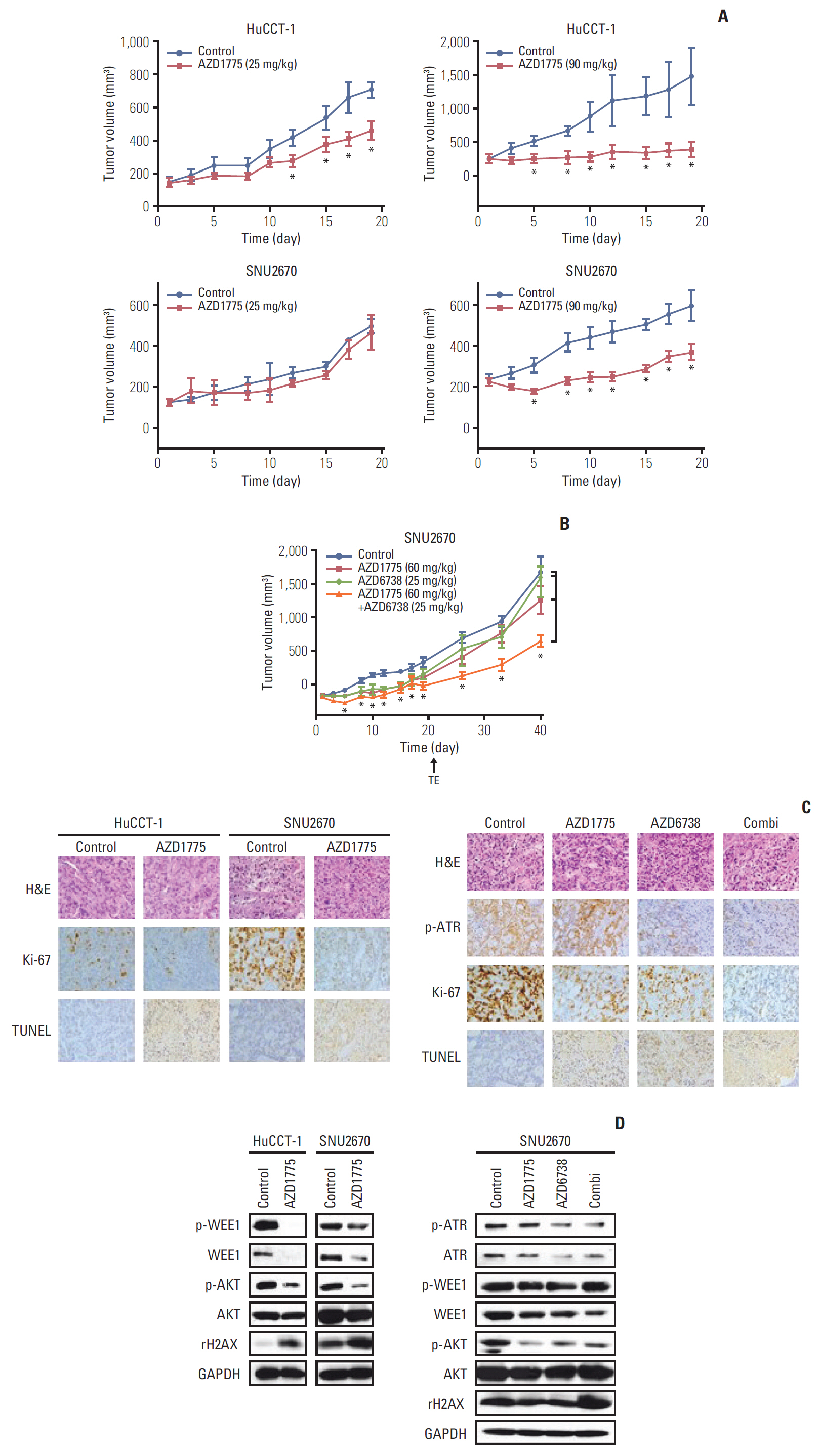
In this study, nine BTC cell lines were exposed to the WEE1 inhibitor (AZD1775). In vitro, MTT assay, colony-forming assay, cell cycle analysis, phospho-histone H3 staining assay, Transwell migration assay, and western blot were performed. Then, to enhance the antitumor effect of AZD1775, the combination treatment of WEE1 inhibitor and ataxia telangiectasia mutated and Rad3 related (ATR) inhibitor (AZD6738) was conducted using MTT assay and comet assay. Finally, HuCCT-1 and SNU2670 xenograft models were established to confirm the anti-tumor effect of AZD1775 alone. Furthermore, the combination treatment was also evaluated in SNU2670 xenograft models.
Results
AZD1775 blocked the phosphorylation of CDC2 and CDC25C in all cell lines, but significantly increased apoptosis and S phase arrest in sensitive cells. However, increased p-ATR and phosphorylated ataxia telangiectasia mutated levels were observed in less sensitive cells. In addition, in vitro and in vivo data illustrated that AZD1775 combined with AZD6738 exerted more potent anti-tumor effects than either drug alone. Although WEE1 inhibition has promising anti-tumor effects in some BTC cells, the addition of ATR inhibitors could enhance its efficacy.
Conclusion
Taken together, this study supports further clinical development of DDR-targeted strategies as monotherapy or combination regimens for BTC.
Keyword
Figure
Cited by 2 articles
-
Enrichment of Wee1/CDC2 and NF-κB Signaling Pathway Constituents Mutually Contributes to CDDP Resistance in Human Osteosarcoma
Zhengbo Hu, Lugen Li, Wenxing Lan, Xiao Wei, Xiangyuan Wen, Penghuan Wu, Xianliao Zhang, Xinhua Xi, Yufa Li, Liqi Wu, Wenhu Li, Xiaohong Liao
Cancer Res Treat. 2022;54(1):277-293. doi: 10.4143/crt.2021.320.Inhibition of WEE1 Potentiates Sensitivity to PARP Inhibitor in Biliary Tract Cancer
Hye-Rim Seo, Ah-Rong Nam, Ju-Hee Bang, Kyoung-Seok Oh, Jae-Min Kim, Jeesun Yoon, Tae-Yong Kim, Do-Youn Oh
Cancer Res Treat. 2022;54(2):541-553. doi: 10.4143/crt.2021.473.
Reference
-
References
1. Pilie PG, Tang C, Mills GB, Yap TA. State-of-the-art strategies for targeting the DNA damage response in cancer. Nat Rev Clin Oncol. 2019; 16:81–104.
Article2. Bogenberger JM, DeLeon TT, Arora M, Ahn DH, Borad MJ. Emerging role of precision medicine in biliary tract cancers. NPJ Precis Oncol. 2018; 2:21.
Article3. Hyung J, Kim B, Yoo C, Kim KP, Jeong JH, Chang HM, et al. Clinical benefit of maintenance therapy for sdvanced biliary tract cancer patients showing no progression after first-line gemcitabine plus cisplatin. Cancer Res Treat. 2019; 51:901–9.4. Nakamura H, Arai Y, Totoki Y, Shirota T, Elzawahry A, Kato M, et al. Genomic spectra of biliary tract cancer. Nat Genet. 2015; 47:1003–10.
Article5. Otto T, Sicinski P. Cell cycle proteins as promising targets in cancer therapy. Nat Rev Cancer. 2017; 17:93–115.
Article6. Matheson CJ, Backos DS, Reigan P. Targeting WEE1 kinase in cancer. Trends Pharmacol Sci. 2016; 37:872–81.
Article7. Geenen JJ, Schellens JH. Molecular pathways: targeting the protein kinase Wee1 in cancer. Clin Cancer Res. 2017; 23:4540–4.
Article8. Krajewska M, Heijink AM, Bisselink YJ, Seinstra RI, Sillje HH, de Vries EG, et al. Forced activation of Cdk1 via wee1 inhibition impairs homologous recombination. Oncogene. 2013; 32:3001–8.
Article9. Richer AL, Cala JM, O'Brien K, Carson VM, Inge LJ, Whitsett TG. WEE1 kinase inhibitor AZD1775 has preclinical efficacy in LKB1-deficient non-small cell lung cancer. Cancer Res. 2017; 77:4663–72.
Article10. Nam AR, Kim JW, Cha Y, Ha H, Park JE, Bang JH, et al. Therapeutic implication of HER2 in advanced biliary tract cancer. Oncotarget. 2016; 7:58007–21.
Article11. Nam AR, Jin MH, Park JE, Bang JH, Oh DY, Bang YJ. Therapeutic targeting of the DNA damage response using an ATR inhibitor in biliary tract cancer. Cancer Res Treat. 2019; 51:1167–79.
Article12. Oku Y, Nishiya N, Tazawa T, Kobayashi T, Umezawa N, Sugawara Y, et al. Augmentation of the therapeutic efficacy of WEE1 kinase inhibitor AZD1775 by inhibiting the YAP-E2F1-DNA damage response pathway axis. FEBS Open Bio. 2018; 8:1001–12.13. Wright G, Golubeva V, Remsing Rix LL, Berndt N, Luo Y, Ward GA, et al. Dual targeting of WEE1 and PLK1 by AZD1775 elicits single agent cellular anticancer activity. ACS Chem Biol. 2017; 12:1883–92.
Article14. Jin MH, Oh DY. ATM in DNA repair in cancer. Pharmacol Ther. 2019; 203:107391.
Article15. Lecona E, Fernandez-Capetillo O. Targeting ATR in cancer. Nat Rev Cancer. 2018; 18:586–95.
Article16. Lin X, Chen D, Zhang C, Zhang X, Li Z, Dong B, et al. Augmented antitumor activity by olaparib plus AZD1775 in gastric cancer through disrupting DNA damage repair pathways and DNA damage checkpoint. J Exp Clin Cancer Res. 2018; 37:129.
Article17. Jin J, Fang H, Yang F, Ji W, Guan N, Sun Z, et al. Combined inhibition of ATR and WEE1 as a novel therapeutic strategy in triple-negative breast cancer. Neoplasia. 2018; 20:478–88.
Article18. Chen D, Lin X, Gao J, Shen L, Li Z, Dong B, et al. Wee1 inhibitor AZD1775 combined with cisplatin potentiates anticancer activity against gastric cancer by increasing DNA damage and cell apoptosis. Biomed Res Int. 2018; 2018:5813292.
Article19. Chen WT, Ebelt ND, Stracker TH, Xhemalce B, Van Den Berg CL, Miller KM. ATM regulation of IL-8 links oxidative stress to cancer cell migration and invasion. Elife. 2015; 4:e07270.
Article20. Zheng H, Shao F, Martin S, Xu X, Deng CX. WEE1 inhibition targets cell cycle checkpoints for triple negative breast cancers to overcome cisplatin resistance. Sci Rep. 2017; 7:43517.
Article21. Ahn DH, Javle M, Ahn CW, Jain A, Mikhail S, Noonan AM, et al. Next-generation sequencing survey of biliary tract cancer reveals the association between tumor somatic variants and chemotherapy resistance. Cancer. 2016; 122:3657–66.
Article22. Sun L, Moore E, Berman R, Clavijo PE, Saleh A, Chen Z, et al. WEE1 kinase inhibition reverses G2/M cell cycle checkpoint activation to sensitize cancer cells to immunotherapy. Oncoimmunology. 2018; 7:e1488359.
Article23. Sun LL, Yang RY, Li CW, Chen MK, Shao B, Hsu JM, et al. Inhibition of ATR downregulates PD-L1 and sensitizes tumor cells to T cell-mediated killing. Am J Cancer Res. 2018; 8:1307–16.24. Kim HJ, Min A, Im SA, Jang H, Lee KH, Lau A, et al. Antitumor activity of the ATR inhibitor AZD6738 in HER2 positive breast cancer cells. Int J Cancer. 2017; 140:109–19.
Article25. Toulany M, Lee KJ, Fattah KR, Lin YF, Fehrenbacher B, Schaller M, et al. Akt promotes post-irradiation survival of human tumor cells through initiation, progression, and termination of DNA-PKcs-dependent DNA double-strand break repair. Mol Cancer Res. 2012; 10:945–57.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhibition of WEE1 Potentiates Sensitivity to PARP Inhibitor in Biliary Tract Cancer
- Therapeutic Co-targeting of WEE1 and ATM Downregulates PD-L1 Expression in Pancreatic Cancer
- Therapeutic Targeting of the DNA Damage Response Using an ATR Inhibitor in Biliary Tract Cancer
- Exploiting DNA replication stress for sequential therapy with PARP and WEE1 inhibitors
- Enrichment of Wee1/CDC2 and NF-κB Signaling Pathway Constituents Mutually Contributes to CDDP Resistance in Human Osteosarcoma