Cancer Res Treat.
2020 Jul;52(3):925-937. 10.4143/crt.2019.533.
Prognostic Value of TP53 Mutation for Transcatheter Arterial Chemoembolization Failure/Refractoriness in HBV-Related Advanced Hepatocellular Carcinoma
- Affiliations
-
- 1Department of Interventional Oncology, the First Affiliated Hospital of Sun Yat-Sen University, Guangzhou, China
- KMID: 2504471
- DOI: http://doi.org/10.4143/crt.2019.533
Abstract
- Purpose
This study aimed to investigate the clinicopathologic features and mutational landscape of patients with hepatitis B virus (HBV)–related advanced hepatocellular carcinomas (HCC) undergoing transcatheter arterial chemoembolization (TACE).
Materials and Methods
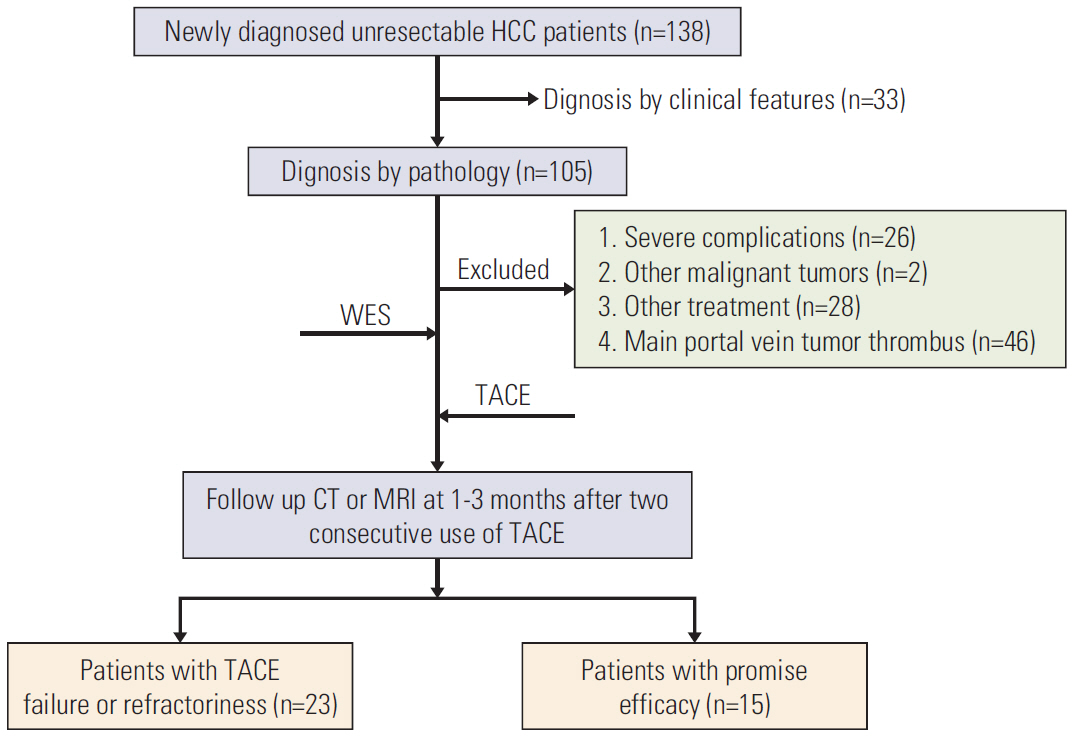
From January 2017 to December 2018, 38 patients newly diagnosed with HBV-related advanced HCC were enrolled in the final analysis. Their pathological tissues and corresponding blood samples before TACE treatment were collected for whole-exome sequencing. Response to TACE was evaluated at 1-3 months after two consecutive use of TACE. Predictive factors were analyzed by univariate and multivariate analyses in a bivariate Logistic regression model. Enrichment of related pathways of all driver genes were acquired using the gene set enrichment analysis (GSEA).
Results
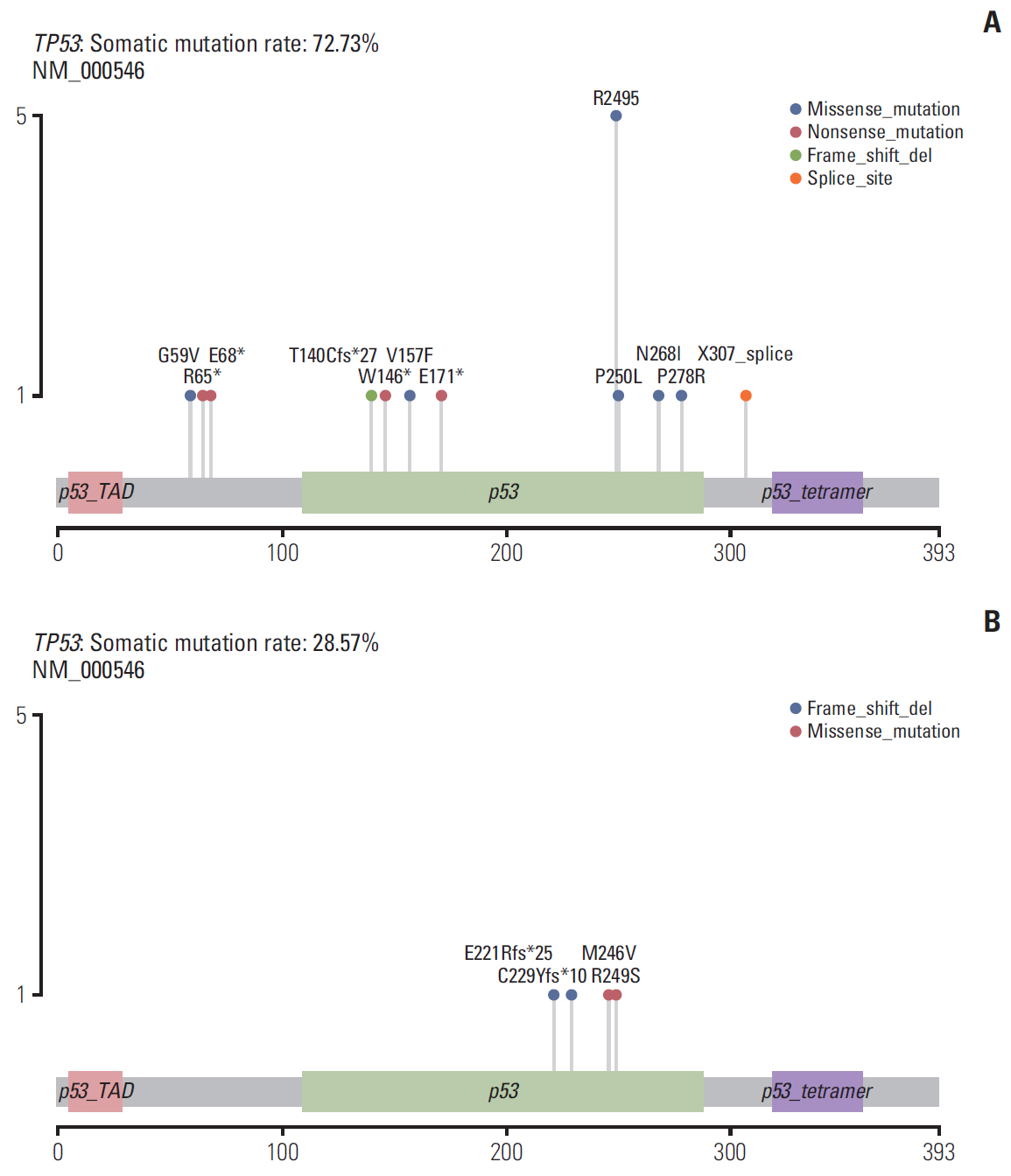
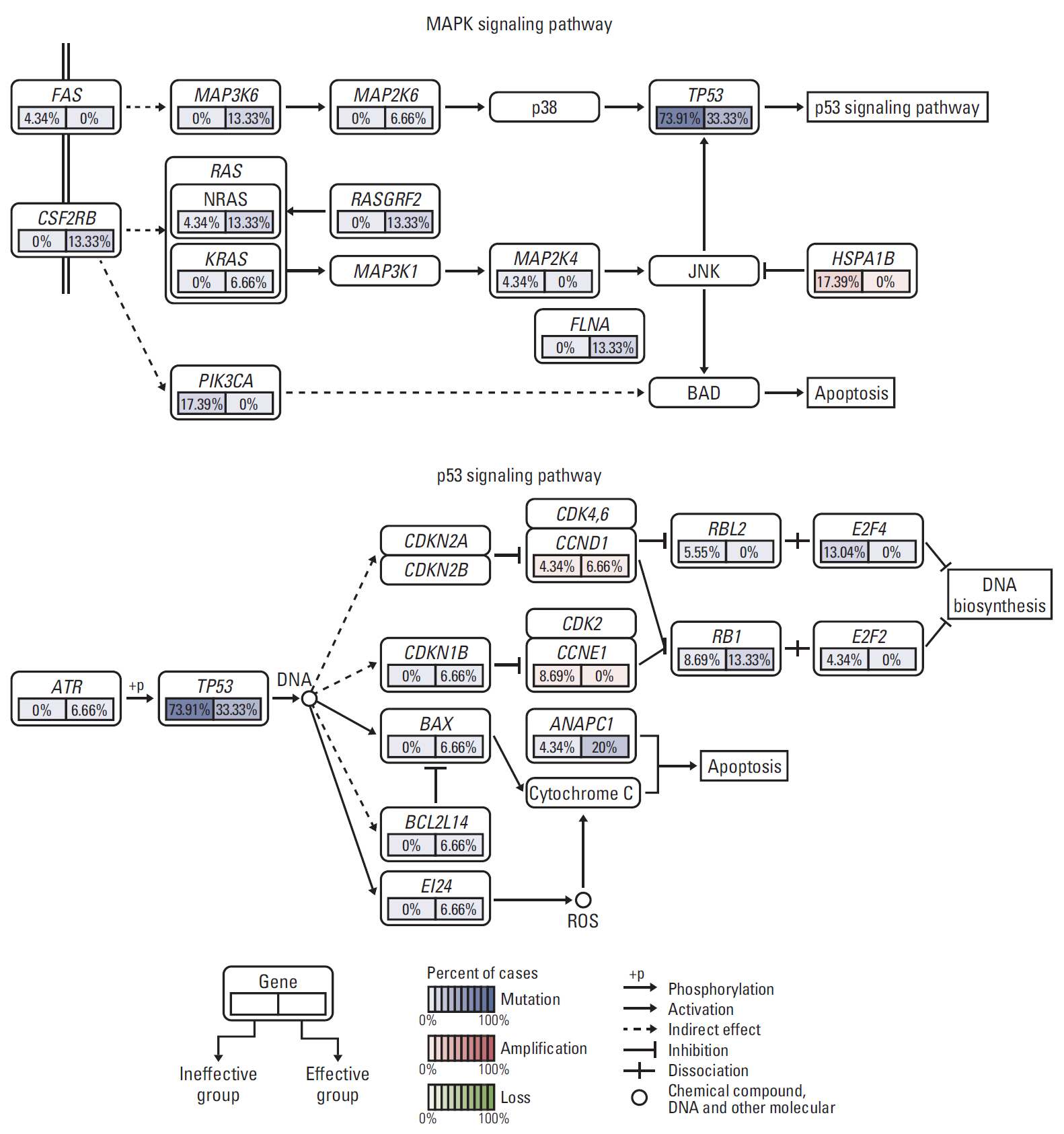
Among 38 patients, 23 (60.5%) exhibited TACE failure/refractoriness. Patients with TACE failure/refractoriness showed higher frequency of TP53 mutation than their counterparts (p=0.020). Univariate and multivariate analyses showed that only vascular invasion and TP53 mutation were significantly correlated with TACE failure/refractoriness in HBV-related advanced HCC. Of the 16 patients without vascular invasion, eight (50.0%) had TP53 mutations, and TP53 mutation was associated with TACE failure/refractoriness (p=0.041). Moreover, GSEA showed that mitogen-activated protein kinase and apoptosis pathways induced by TP53 mutation were possibly associated with TACE failure/refractoriness.
Conclusion
Our study suggested that TP53 mutation was independently related with TACE efficacy, which may work via mitogen-activated protein kinase and apoptosis pathways. These findings may provide evidence to help distinguish patients who will particularly benefit from TACE from those who require more personalized therapeutic regimens and rigorous surveillance in HBV-related advanced HCC.
Keyword
Figure
Reference
-
References
1. El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007; 132:2557–76.
Article2. Lencioni R, de Baere T, Soulen MC, Rilling WS, Geschwind JF. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: a systematic review of efficacy and safety data. Hepatology. 2016; 64:106–16.
Article3. Fan J, Zhou J, Wang JH, Qing SK, Zeng MS, Cong WM, et al. Guidance for diagnosis and treatment of primary liver cancer. Chin J Pract Surg. 2017; 16:705–20.4. Vesselle G, Quirier-Leleu C, Velasco S, Charier F, Silvain C, Boucebci S, et al. Predictive factors for complete response of chemoembolization with drug-eluting beads (DEB-TACE) for hepatocellular carcinoma. Eur Radiol. 2016; 26:1640–8.
Article5. Jeong SO, Kim EB, Jeong SW, Jang JY, Lee SH, Kim SG, et al. Predictive factors for complete response and recurrence after transarterial chemoembolization in hepatocellular carcinoma. Gut Liver. 2017; 11:409–16.
Article6. Kudo M, Matsui O, Izumi N, Kadoya M, Okusaka T, Miyayama S, et al. Transarterial chemoembolization failure/refractoriness: JSH-LCSGJ criteria 2014 update. Oncology. 2014; 87 Suppl 1:22–31.
Article7. Zucman-Rossi J, Villanueva A, Nault JC, Llovet JM. Genetic landscape and biomarkers of hepatocellular carcinoma. Gastroenterology. 2015; 149:1226–39.
Article8. Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019; 380:1450–62.
Article9. Nault JC, Martin Y, Caruso S, Hirsch TZ, Bayard Q, Calderaro J, et al. Clinical impact of genomic diversity from early to advanced hepatocellular carcinoma. Hepatology. 2020; 71:164–82.
Article10. Woo HG, Wang XW, Budhu A, Kim YH, Kwon SM, Tang ZY, et al. Association of TP53 mutations with stem cell-like gene expression and survival of patients with hepatocellular carcinoma. Gastroenterology. 2011; 140:1063–70.
Article11. Liu Q, Liu N, Shangguan Q, Zhang F, Chai W, Tong X, et al. LncRNA SAMD12-AS1 promotes cell proliferation and inhibits apoptosis by interacting with NPM1. Sci Rep. 2019; 9:11593.
Article12. Kamat CD, Green DE, Warnke L, Thorpe JE, Ceriello A, Ihnat MA. Mutant p53 facilitates pro-angiogenic, hyperproliferative phenotype in response to chronic relative hypoxia. Cancer Lett. 2007; 249:209–19.
Article13. Mizuarai S, Yamanaka K, Kotani H. Mutant p53 induces the GEF-H1 oncogene, a guanine nucleotide exchange factor-H1 for RhoA, resulting in accelerated cell proliferation in tumor cells. Cancer Res. 2006; 66:6319–26.
Article14. Speidel D. The role of DNA damage responses in p53 biology. Arch Toxicol. 2015; 89:501–17.
Article15. Gouas DA, Villar S, Ortiz-Cuaran S, Legros P, Ferro G, Kirk GD, et al. TP53 R249S mutation, genetic variations in HBX and risk of hepatocellular carcinoma in The Gambia. Carcinogenesis. 2012; 33:1219–24.
Article16. Liao P, Zeng SX, Zhou X, Chen T, Zhou F, Cao B, et al. Mutant p53 gains its function via c-Myc activation upon CDK4 phosphorylation at serine 249 and consequent PIN1 binding. Mol Cell. 2017; 68:1134–46.
Article17. Chen ZX, Jian ZW, Wu XW, Wang JC, Peng JY, Lao XM. Clinical conditions and treatment requirements for long-term survival among hepatitis B-related hepatocellular carcinoma initially treated with chemoembolization. Cancer Med. 2019; 8:5097–107.
Article18. Raoul JL, Forner A, Bolondi L, Cheung TT, Kloeckner R, de Baere T. Updated use of TACE for hepatocellular carcinoma treatment: How and when to use it based on clinical evidence. Cancer Treat Rev. 2019; 72:28–36.
Article19. Oda T, Tsuda H, Scarpa A, Sakamoto M, Hirohashi S. Mutation pattern of the p53 gene as a diagnostic marker for multiple hepatocellular carcinoma. Cancer Res. 1992; 52:3674–8.20. Dimri M, Humphries A, Laknaur A, Elattar S, Lee TJ, Sharma A, et al. NAD(P)H quinone dehydrogenase 1 ablation inhibits activation of the phosphoinositide 3-kinase/Akt serine/threonine kinase and mitogen-activated protein kinase/extracellular signal-regulated kinase pathways and blocks metabolic adaptation in hepatocellular carcinoma. Hepatology. 2020; 71:549–68.
Article21. Chen L, Guo P, He Y, Chen Z, Chen L, Luo Y, et al. HCCderived exosomes elicit HCC progression and recurrence by epithelial-mesenchymal transition through MAPK/ERK signalling pathway. Cell Death Dis. 2018; 9:513.
Article22. Xu G, Zhu L, Wang Y, Shi Y, Gong A, Wu C. Stattic enhances radiosensitivity and reduces radio-induced migration and invasion in HCC cell lines through an apoptosis pathway. Biomed Res Int. 2017; 2017:1832494.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Fatal Case of Pulmonary Embolism after Transcatheter Arterial Chemoembolization for Hepatocellular Carcinoma
- Rupture of hepatocellular carcinoma after transcatheter arterial chemoembolization: A case report
- Palliative Transcatheter Arterial Chemoembolization for Relieving Metastatic Bone Pain due to Hepatocellular Carcinoma: A Case Report
- Hepatocellular Carcinoma Extending to the Inferior Vena Cava and Right Atrium-A Case Report of 4 Years Survival after Repeated Transcatheter Arterial Chemoembolization Therapy -
- Hepatocellular Carcinoma after Transcatheter Arterial Chemoembolization: Difficulties on Imaging Follow-up