Cancer Res Treat.
2020 Jul;52(3):815-829. 10.4143/crt.2019.380.
G Protein–Coupled Receptor 30 Mediates the Anticancer Effects Induced by Eicosapentaenoic Acid in Ovarian Cancer Cells
- Affiliations
-
- 1State Key Laboratory of Pharmaceutical Biotechnology and Jiangsu Key Laboratory of Molecular Medicine, Medical School of Nanjing University, Nanjing, China
- 2Department of Obstetrics and Gynecology, Xiamen Chang Gung Hospital, Xiamen, China
- 3Obstetrics and Gynecology Hospital, Fudan University, Shanghai, China
- 4Department of Pathology, Nanjing Drum Tower Hospital, The Affiliated Hospital of Nanjing University Medical School, Nanjing, China
- 5Biology Science Institutes, Chongqing Medical University, Yuzhong, China
- KMID: 2504462
- DOI: http://doi.org/10.4143/crt.2019.380
Abstract
- Purpose
While numerous epidemiological studies have indicated that omega-3 polyunsaturated fatty acids have anticancer properties in various cancers, the effects and mechanisms of eicosapentaenoic acid (EPA) in ovarian cancer cell growth are poorly understood.
Materials and Methods
ES2 ovarian clear cell carcinoma cells and SKOV3 adenocarcinoma cells were treated with palmitic acid or EPA, followed by flow cytometry and cell counting to measure apoptosis and proliferation, respectively. A modified protein lipid overlay assay was used to further verify whether EPA was a ligand of G protein–coupled receptor 30 (GPR30) in ES2 cells. The levels of apoptosis-related genes, phosphorylated AKT, and phosphorylated ERK1/2 were detected to explore the underlying mechanism. Finally, inhibitory effect of EPA on tumor growth via GPR30 was determined in vitro and in vivo.
Results
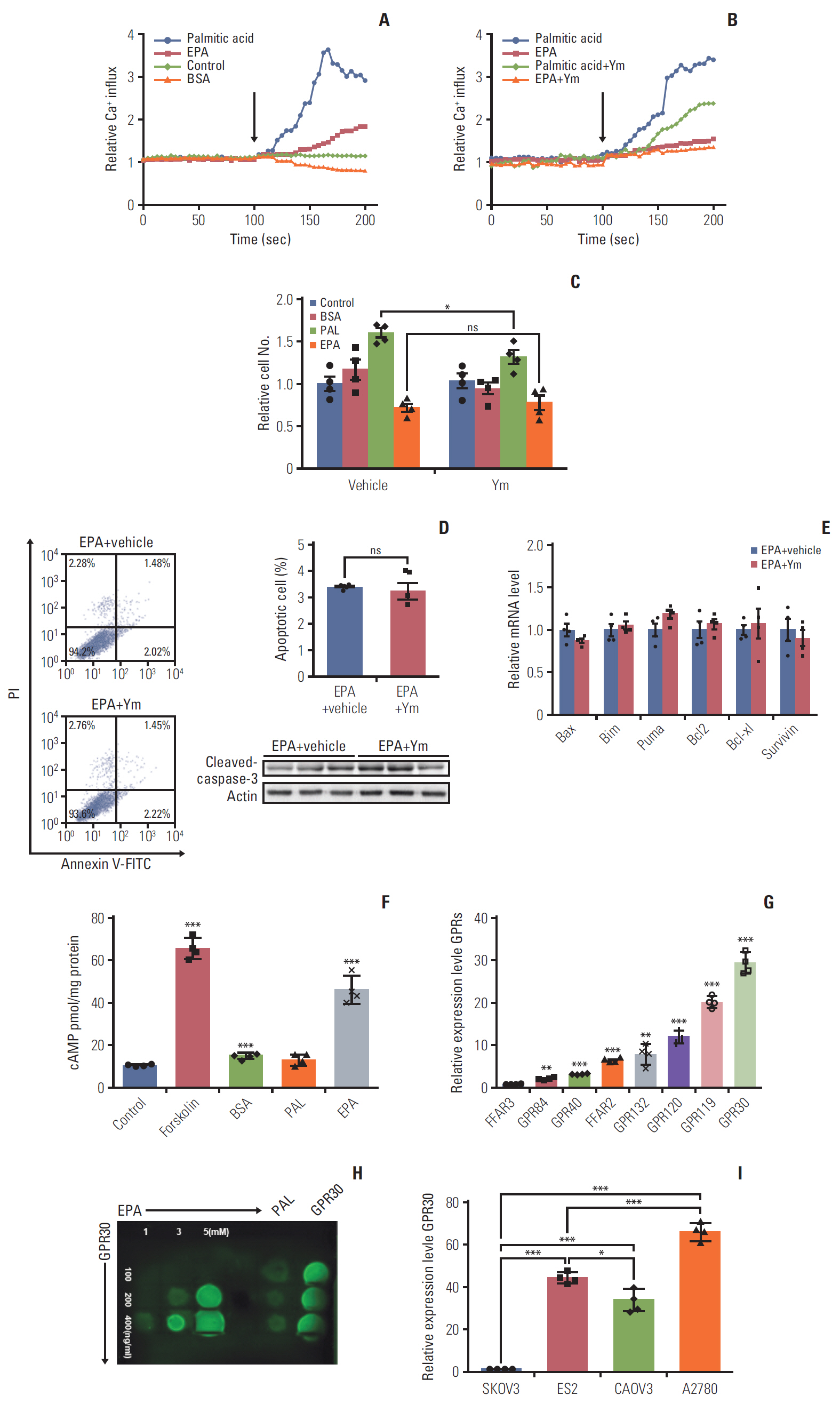
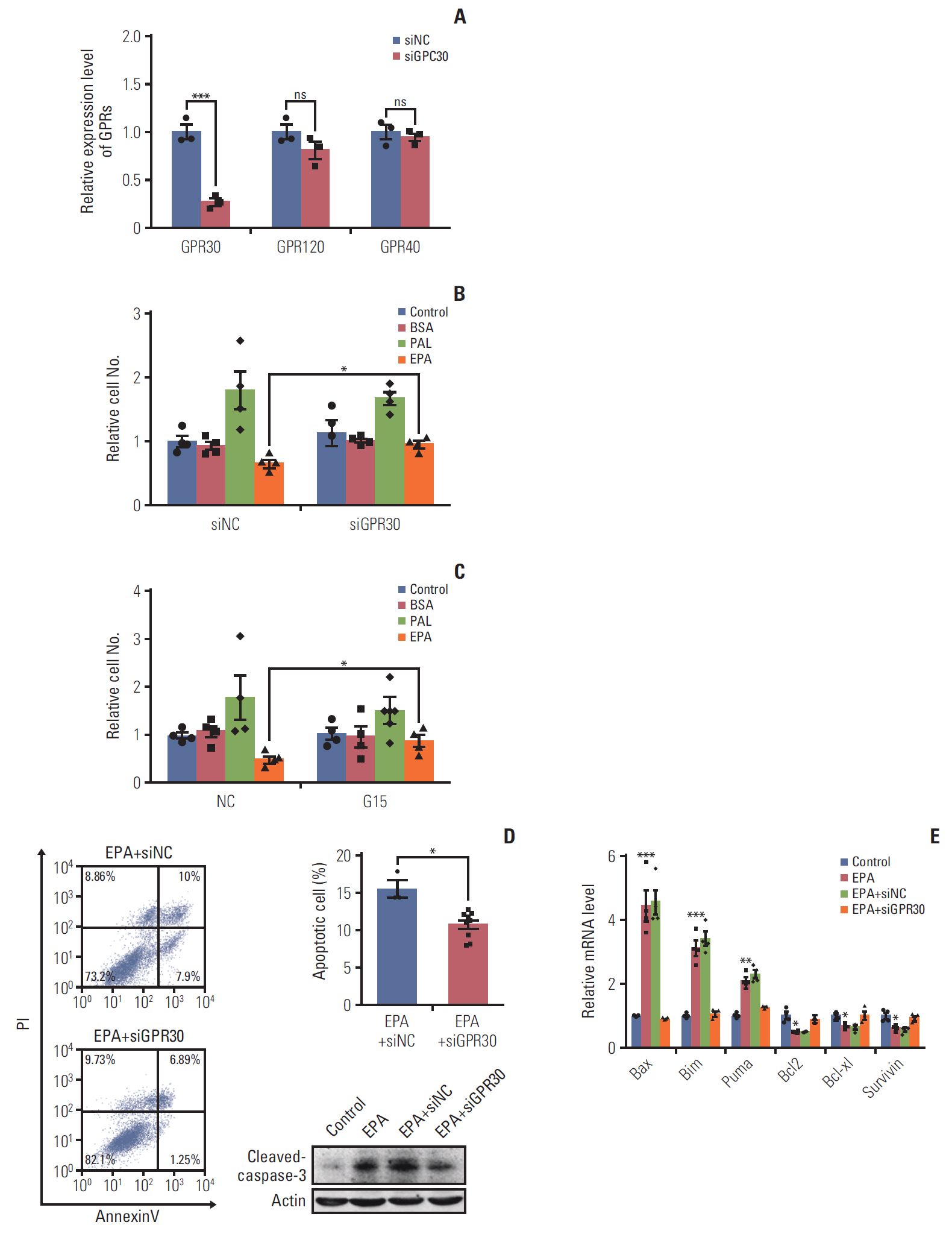
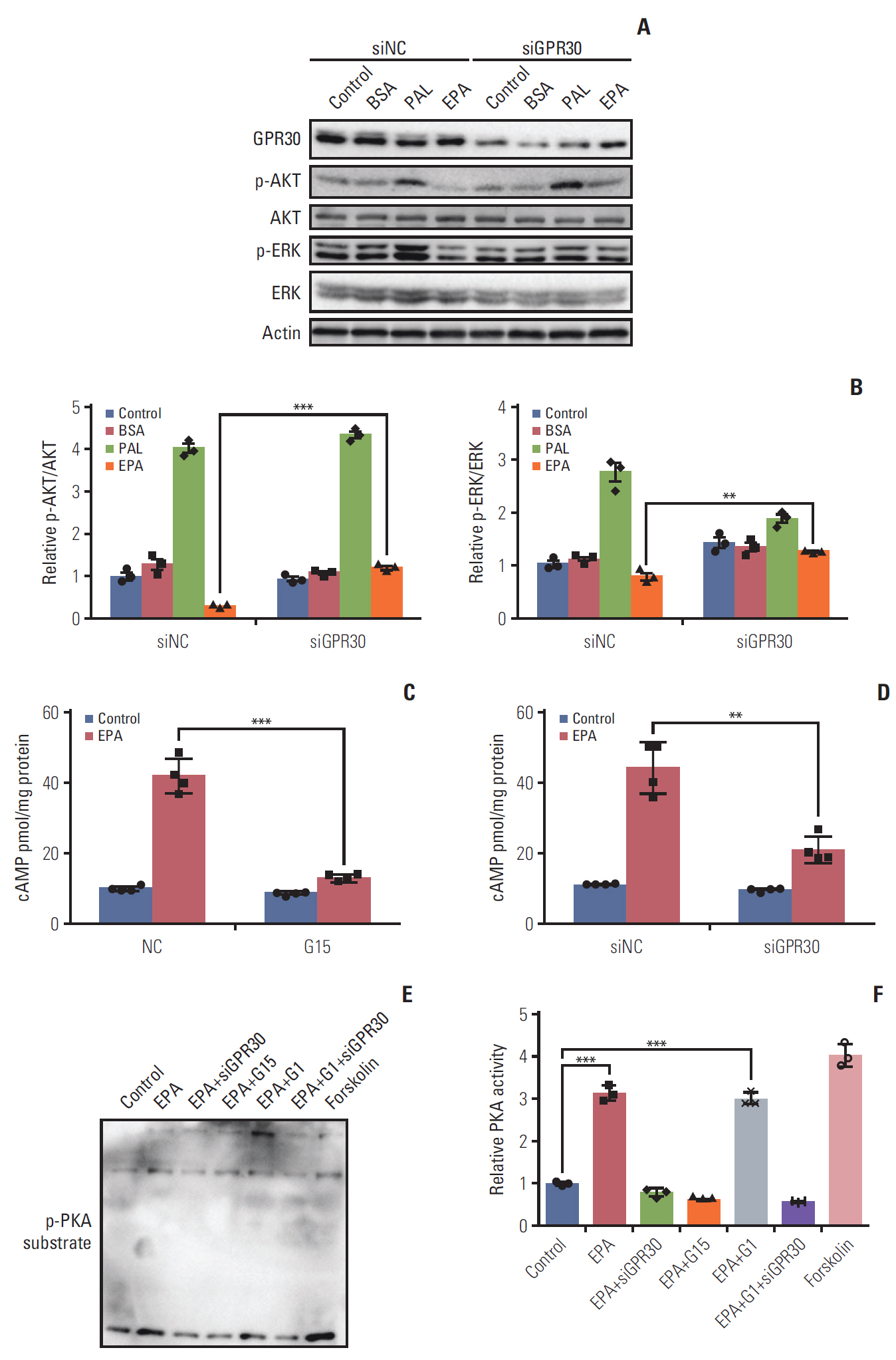
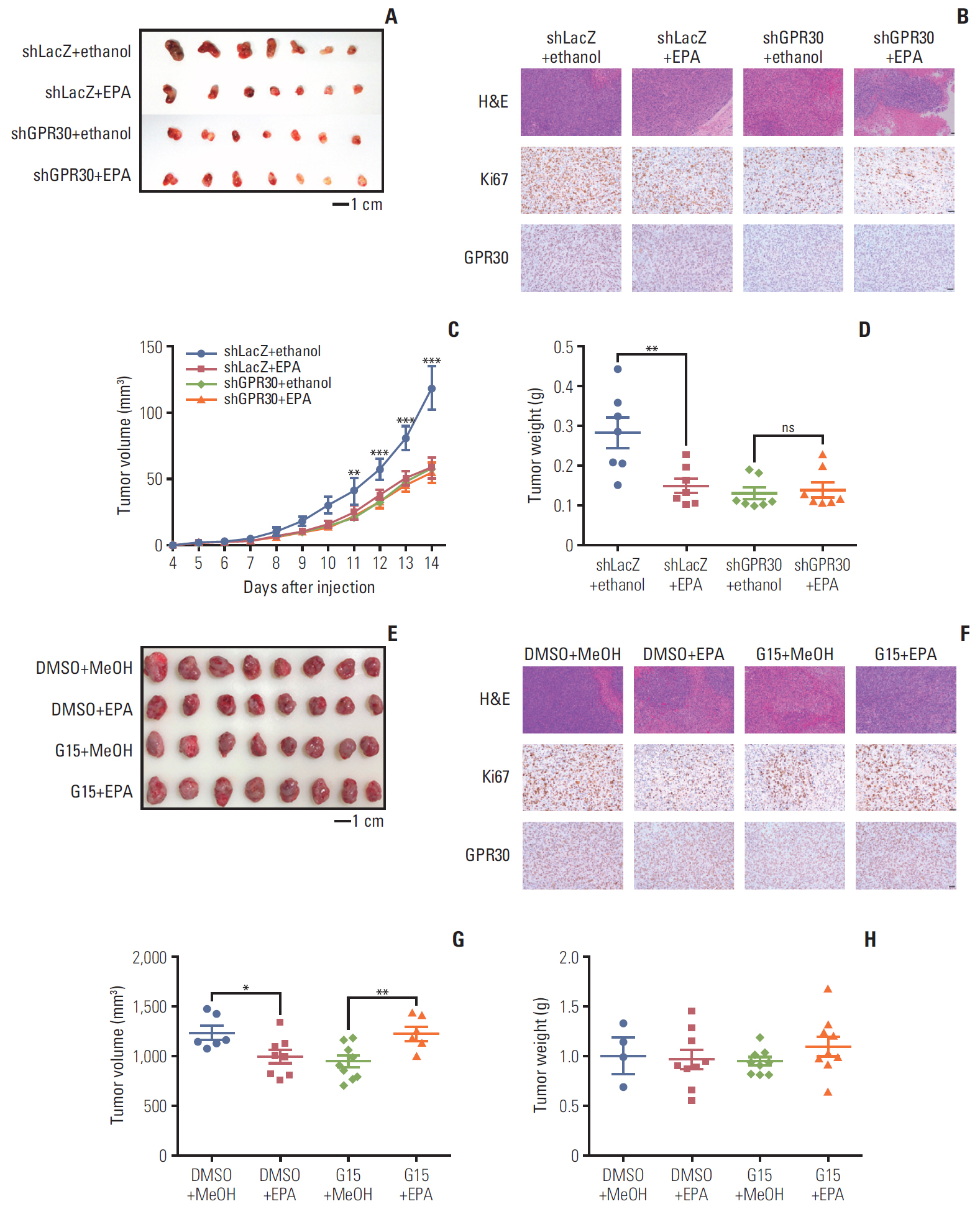
EPA suppressed ES2 ovarian clear cell carcinoma cells growth via GPR30, a novel EPA receptor, by inducing apoptosis. As a ligand of GPR30, EPA activated the GPR30-cAMP– protein kinase A signaling pathway. When GPR30 was suppressed by siRNA or its inhibitor G15, the antiproliferative action of EPA was impaired. Furthermore, EPA inhibited tumor growth by blocking the activation of AKT and ERK. In the mouse xenograft model, EPA decreased tumor volume and weight through GPR30 by blocking tumor cell proliferation.
Conclusion
These results confirm that EPA is a tumor suppressor in human ovarian clear cell carcinoma cells and functions through a novel fatty acid receptor, GPR30, indicating a mechanistic linkage between omega-3 fatty acids and cancers.
Figure
Reference
-
References
1. Sapienza C, Issa JP. Diet, nutrition, and cancer epigenetics. Annu Rev Nutr. 2016; 36:665–81.
Article2. Rose DP, Connolly JM. Omega-3 fatty acids as cancer chemopreventive agents. Pharmacol Ther. 1999; 83:217–44.
Article3. Velentzis LS, Keshtgar MR, Woodside JV, Leathem AJ, Titcomb A, Perkins KA, et al. Significant changes in dietary intake and supplement use after breast cancer diagnosis in a UK multicentre study. Breast Cancer Res Treat. 2011; 128:473–82.
Article4. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010; 60:277–300.
Article5. Brinton EA, Mason RP. Prescription omega-3 fatty acid products containing highly purified eicosapentaenoic acid (EPA). Lipids Health Dis. 2017; 16:23.
Article6. Santos CR, Schulze A. Lipid metabolism in cancer. FEBS J. 2012; 279:2610–23.
Article7. Maggiolini M, Picard D. The unfolding stories of GPR30, a new membrane-bound estrogen receptor. J Endocrinol. 2010; 204:105–14.
Article8. Heublein S, Mayr D, Friese K, Jarrin-Franco MC, Lenhard M, Mayerhofer A, et al. The G-protein-coupled estrogen receptor (GPER/GPR30) in ovarian granulosa cell tumors. Int J Mol Sci. 2014; 15:15161–72.
Article9. Mazzuca MQ, Mata KM, Li W, Rangan SS, Khalil RA. Estrogen receptor subtypes mediate distinct microvascular dilation and reduction in [Ca2+]I in mesenteric microvessels of female rat. J Pharmacol Exp Ther. 2015; 352:291–304.
Article10. Jin J, Mao Y, Thomas D, Kim S, Daniel JL, Kunapuli SP. RhoA downstream of G(q) and G(12/13) pathways regulates protease-activated receptor-mediated dense granule release in platelets. Biochem Pharmacol. 2009; 77:835–44.
Article11. Taniguchi M, Nagai K, Arao N, Kawasaki T, Saito T, Moritani Y, et al. YM-254890, a novel platelet aggregation inhibitor produced by Chromobacterium sp. QS3666. J Antibiot (Tokyo). 2003; 56:358–63.
Article12. Wang C, Lv X, Jiang C, Davis JS. The putative G-protein coupled estrogen receptor agonist G-1 suppresses proliferation of ovarian and breast cancer cells in a GPER-independent manner. Am J Transl Res. 2012; 4:390–402.13. Bai LY, Weng JR, Hu JL, Wang D, Sargeant AM, Chiu CF. G15, a GPR30 antagonist, induces apoptosis and autophagy in human oral squamous carcinoma cells. Chem Biol Interact. 2013; 206:375–84.
Article14. Pal D, Dasgupta S, Kundu R, Maitra S, Das G, Mukhopadhyay S, et al. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat Med. 2012; 18:1279–85.
Article15. Hu L, Lau SH, Tzang CH, Wen JM, Wang W, Xie D, et al. Association of Vimentin overexpression and hepatocellular carcinoma metastasis. Oncogene. 2004; 23:298–302.
Article16. Sugiyama T, Kamura T, Kigawa J, Terakawa N, Kikuchi Y, Kita T, et al. Clinical characteristics of clear cell carcinoma of the ovary: a distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy. Cancer. 2000; 88:2584–9.17. Landrum LM, Java J, Mathews CA, Lanneau GS Jr, Copeland LJ, Armstrong DK, et al. Prognostic factors for stage III epithelial ovarian cancer treated with intraperitoneal chemotherapy: a Gynecologic Oncology Group study. Gynecol Oncol. 2013; 130:12–8.
Article18. Kwan HY, Fu X, Liu B, Chao X, Chan CL, Cao H, et al. Subcutaneous adipocytes promote melanoma cell growth by activating the Akt signaling pathway: role of palmitic acid. J Biol Chem. 2014; 289:30525–37.19. Sun H, Hu Y, Gu Z, Owens RT, Chen YQ, Edwards IJ. Omega3 fatty acids induce apoptosis in human breast cancer cells and mouse mammary tissue through syndecan-1 inhibition of the MEK-Erk pathway. Carcinogenesis. 2011; 32:1518–24.
Article20. Kotarsky K, Nilsson NE, Flodgren E, Owman C, Olde B. A human cell surface receptor activated by free fatty acids and thiazolidinedione drugs. Biochem Biophys Res Commun. 2003; 301:406–10.
Article21. Gu Z, Suburu J, Chen H, Chen YQ. Mechanisms of omega-3 polyunsaturated fatty acids in prostate cancer prevention. Biomed Res Int. 2013; 2013:824563.
Article22. Dorsam RT, Gutkind JS. G-protein-coupled receptors and cancer. Nat Rev Cancer. 2007; 7:79–94.
Article23. Prossnitz ER, Oprea TI, Sklar LA, Arterburn JB. The ins and outs of GPR30: a transmembrane estrogen receptor. J Steroid Biochem Mol Biol. 2008; 109:350–3.
Article24. Shu YJ, Weng H, Ye YY, Hu YP, Bao RF, Cao Y, et al. SPOCK1 as a potential cancer prognostic marker promotes the proliferation and metastasis of gallbladder cancer cells by activating the PI3K/AKT pathway. Mol Cancer. 2015; 14:12.
Article25. Brazil DP, Yang ZZ, Hemmings BA. Advances in protein kinase B signalling: AKTion on multiple fronts. Trends Biochem Sci. 2004; 29:233–42.
Article26. Castoria G, Migliaccio A, D'Amato L, Di Stasio R, Ciociola A, Lombardi M, et al. Integrating signals between cAMP and MAPK pathways in breast cancer. Front Biosci. 2008; 13:1318–27.
Article27. Nieman KM, Kenny HA, Penicka CV, Ladanyi A, Buell-Gutbrod R, Zillhardt MR, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011; 17:1498–503.
Article28. Fukui M, Kang KS, Okada K, Zhu BT. EPA, an omega-3 fatty acid, induces apoptosis in human pancreatic cancer cells: role of ROS accumulation, caspase-8 activation, and autophagy induction. J Cell Biochem. 2013; 114:192–203.
Article29. Sabharwal SS, Schumacker PT. Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles' heel? Nat Rev Cancer. 2014; 14:709–21.
Article30. Gajate C, Gonzalez-Camacho F, Mollinedo F. Lipid raft connection between extrinsic and intrinsic apoptotic pathways. Biochem Biophys Res Commun. 2009; 380:780–4.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Stimulatory Anticancer Effect of Resveratrol Mediated by G Protein-Coupled Estrogen Receptor in Colorectal Cancer
- Action and Signaling of Lysophosphatidylethanolamine in MDA-MB-231 Breast Cancer Cells
- Growth Regulation of Ovarian Cancer Cells through the Inactivation of AP-1 by Retinoid Derivatives
- Differential Gene Expression in GPR40-Overexpressing Pancreatic beta-cells Treated with Linoleic Acid
- Anticancer Effect and Apoptosis of All-trans-retinoic Acid on the Human Ovarian Epithelial Carcinoma Cell Lines