Int J Stem Cells.
2020 Jul;13(2):237-245. 10.15283/ijsc20001.
SOCS1 Regulates the Immunomodulatory Roles of MSCs on B Cells
- Affiliations
-
- 1Animal Physiology Laboratory, School of Agroforestry Engineering and Planning, Tongren University, Tongren, China
- 2Department of Neural Engineering and Biological Interdisciplinary Studies, Institute of Military Cognition and Brain Sciences, Academy of Military Medical Sciences, Beijing, China
- 3Department of Anatomy, School of Basic Medical Sciences, Xiangnan University, Chenzhou, China
- 4Department of Geriatrics, Peking University Shenzhen Hospital, Shenzhen, China
- 5Laboratory of Animal Genetic Breeding and Reproduction, Yanbian University, Yanji, China
- KMID: 2504336
- DOI: http://doi.org/10.15283/ijsc20001
Abstract
- Background and Objectives
The effective use of MSCs for the treatment of some B cell-mediated immune diseases is quite limited. The main reason is that the immunomodulatory effects of mesenchymal stem cells (MSCs) on B cells are unclear, and their underlying mechanisms have not been fully explored.
Methods and Results
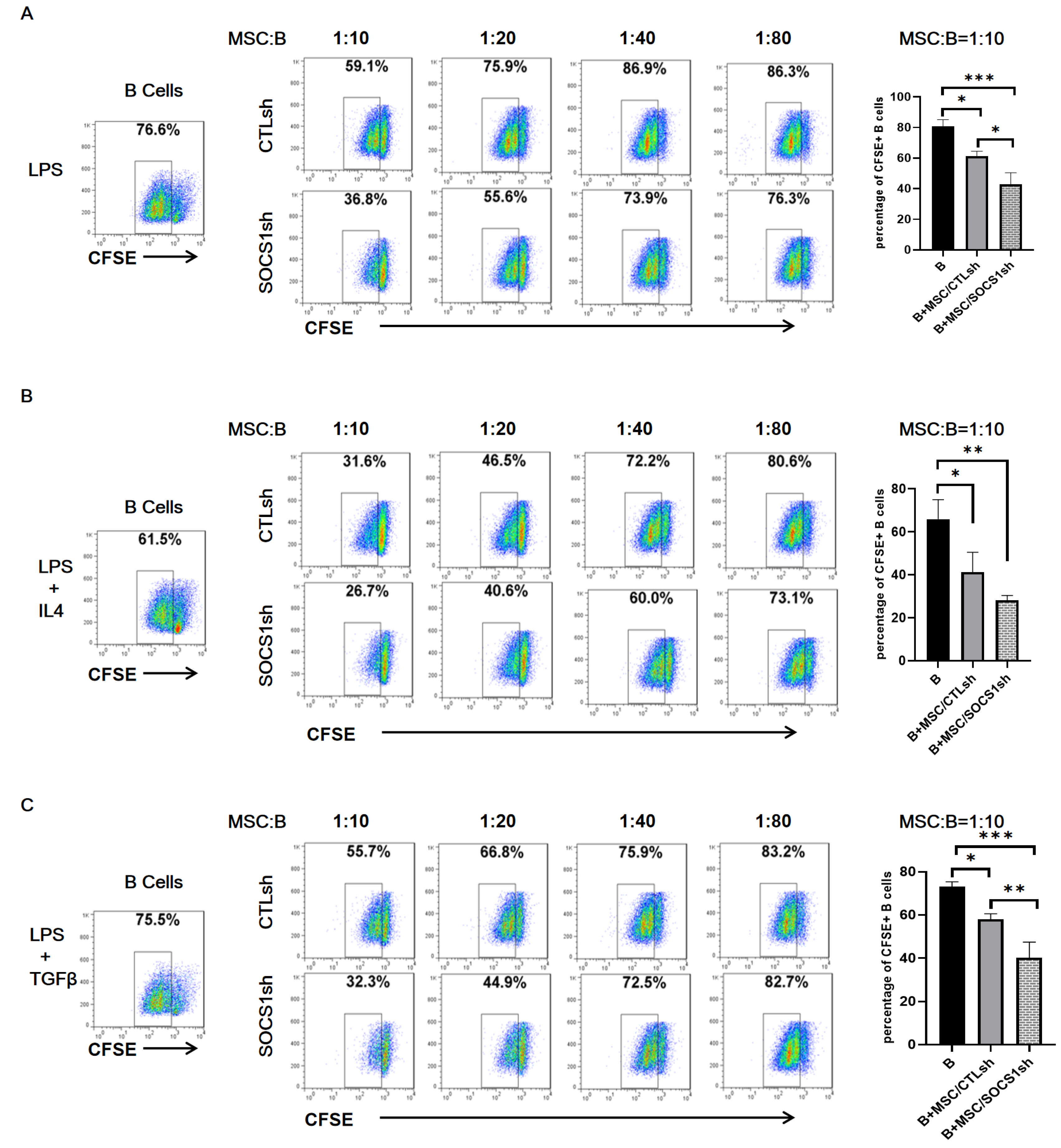
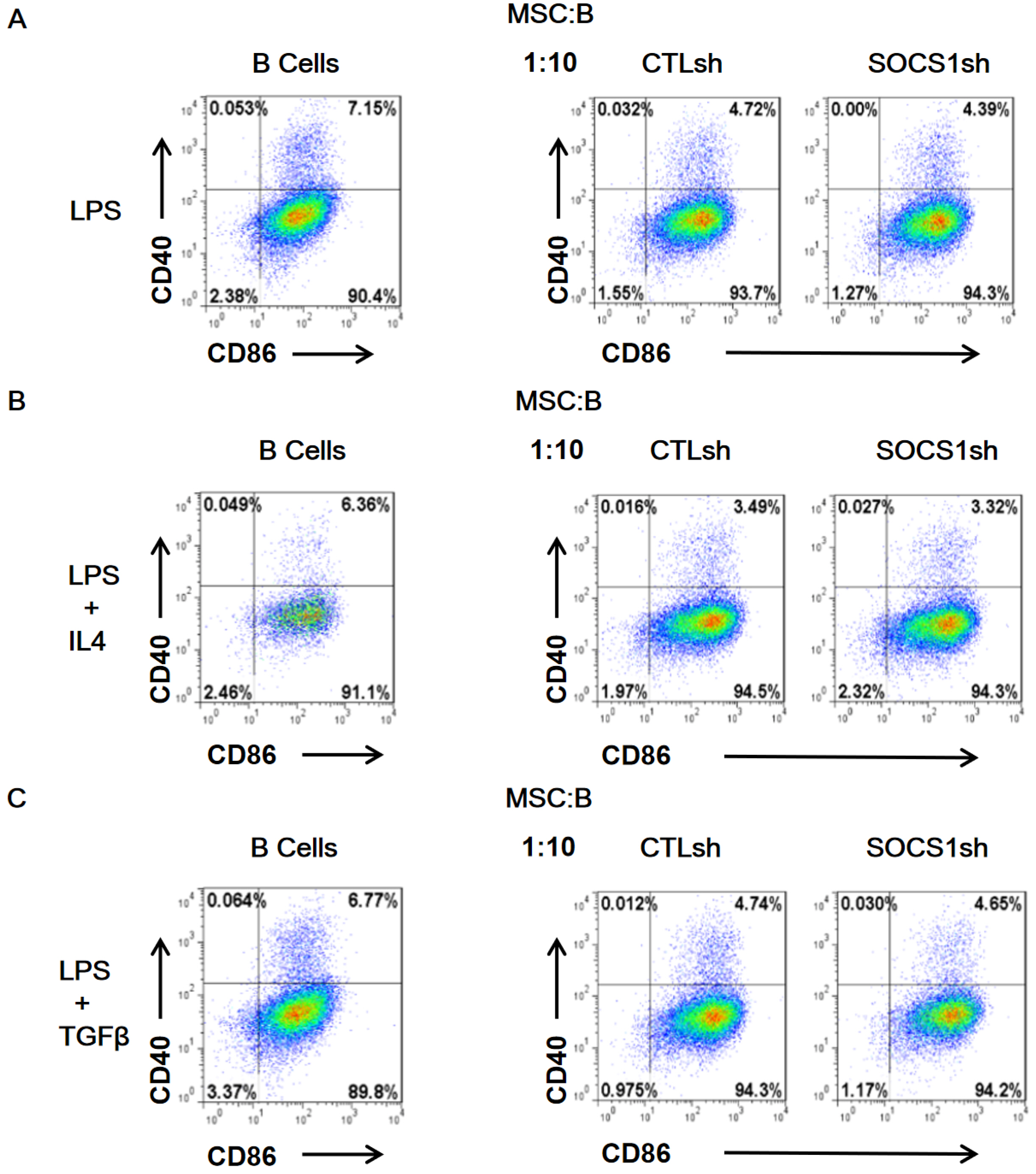
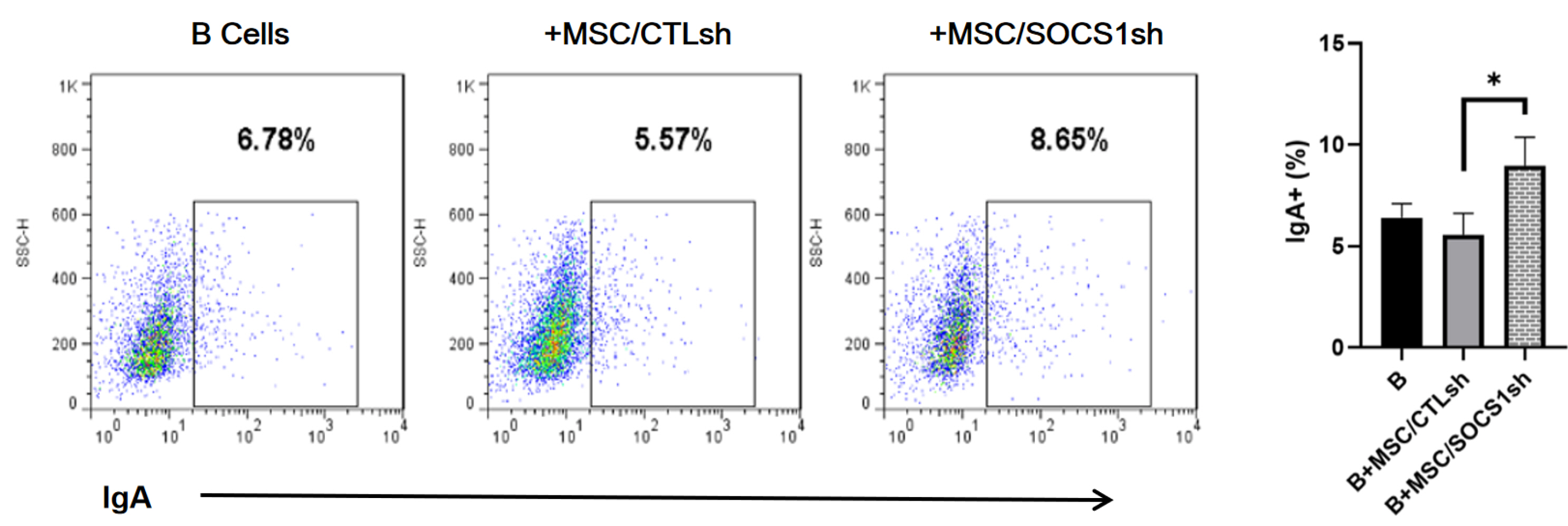
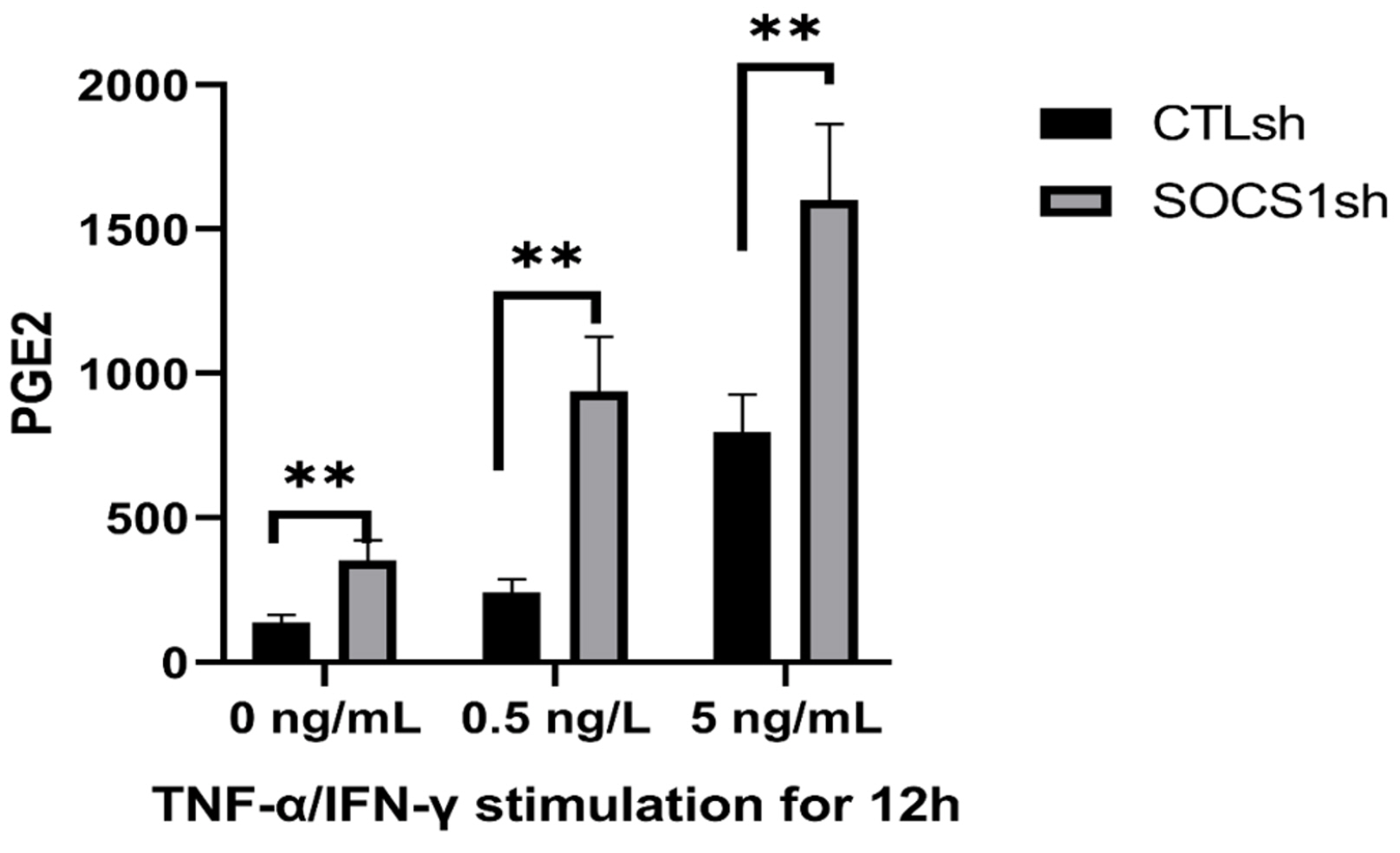
By co-culturing B cells with MSCs without (MSC/CTLsh) or with suppressor of cytokine signaling 1 (SOCS1) knockdown (MSC/SOCS1sh), we found that MSCs inhibited B cell proliferation, activation and terminal differentiation. Remarkably, the highest inhibition of B cell proliferation was observed in MSC/SOCS1sh co-culture. Besides, MSC/SOCS1sh reversed the inhibitory effect of MSCs in the last stage of B cell differentiation. However, MSC/SOCS1sh had no effect on inhibiting B cell activation by MSCs. We also showed that IgA+ B cell production was significantly higher in MSC/SOCS1sh than in MSC/CTLsh, although no difference was observed when both MSCs co-cultures were compared to isolated B cells. In addition, MSCs increased PGE2 production after TNF-α/IFN-γ stimulation, with the highest increase observed in MSC/SOCS1sh co-culture.
Conclusions
Our results highlighted the role of SOCS1 as an important new mediator in the regulation of B cell function by MSCs. Therefore, these data may help to develop new treatments for B cell-mediated immune diseases.
Keyword
Figure
Reference
-
References
1. Wang Y, Chen X, Cao W, Shi Y. 2014; Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. 15:1009–1016. DOI: 10.1038/ni.3002. PMID: 25329189.
Article2. Franquesa M, Hoogduijn MJ, Bestard O, Grinyó JM. 2012; Immunomodulatory effect of mesenchymal stem cells on B cells. Front Immunol. 3:212. DOI: 10.3389/fimmu.2012.00212. PMID: 22833744. PMCID: PMC3400888.
Article3. Bernardo ME, Fibbe WE. 2013; Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell. 13:392–402. DOI: 10.1016/j.stem.2013.09.006. PMID: 24094322.
Article4. Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y. 2008; Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2:141–150. DOI: 10.1016/j.stem.2007.11.014. PMID: 18371435.
Article5. Akiyama K, Chen C, Wang D, Xu X, Qu C, Yamaza T, Cai T, Chen W, Sun L, Shi S. 2012; Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell. 10:544–555. DOI: 10.1016/j.stem.2012.03.007. PMID: 22542159. PMCID: PMC3348385.
Article6. Fan L, Hu C, Chen J, Cen P, Wang J, Li L. 2016; Interaction between mesenchymal stem cells and B-cells. Int J Mol Sci. 17:E650. DOI: 10.3390/ijms17050650. PMID: 27164080. PMCID: PMC4881476.
Article7. Bonnaure G, Gervais-St-Amour C, Néron S. 2016; Bone marrow mesenchymal stem cells enhance the differentiation of human switched memory B lymphocytes into plasma cells in serum-free medium. J Immunol Res. 2016:7801781. DOI: 10.1155/2016/7801781. PMID: 27872867. PMCID: PMC5107863.
Article8. Ji YR, Yang ZX, Han ZB, Meng L, Liang L, Feng XM, Yang SG, Chi Y, Chen DD, Wang YW, Han ZC. 2012; Mesenchymal stem cells support proliferation and terminal differentiation of B cells. Cell Physiol Biochem. 30:1526–1537. DOI: 10.1159/000343340. PMID: 23235695.
Article9. Traggiai E, Volpi S, Schena F, Gattorno M, Ferlito F, Moretta L, Martini A. 2008; Bone marrow-derived mesenchymal stem cells induce both polyclonal expansion and differentiation of B cells isolated from healthy donors and systemic lupus erythematosus patients. Stem Cells. 26:562–569. DOI: 10.1634/stemcells.2007-0528. PMID: 18024418.
Article10. Asari S, Itakura S, Ferreri K, Liu CP, Kuroda Y, Kandeel F, Mullen Y. 2009; Mesenchymal stem cells suppress B-cell terminal differentiation. Exp Hematol. 37:604–615. DOI: 10.1016/j.exphem.2009.01.005. PMID: 19375651. PMCID: PMC2747661.
Article11. Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi GL, Pistoia V, Uccelli A. 2006; Human mesenchymal stem cells modulate B-cell functions. Blood. 107:367–372. DOI: 10.1182/blood-2005-07-2657. PMID: 16141348.
Article12. Luk F, Carreras-Planella L, Korevaar SS, de Witte SFH, Borràs FE, Betjes MGH, Baan CC, Hoogduijn MJ, Franquesa M. 2017; Inflammatory conditions dictate the effect of mesenchymal stem or stromal cells on B cell function. Front Immunol. 8:1042. DOI: 10.3389/fimmu.2017.01042. PMID: 28894451. PMCID: PMC5581385.
Article13. Che N, Li X, Zhang L, Liu R, Chen H, Gao X, Shi S, Chen W, Sun L. 2014; Impaired B cell inhibition by lupus bone marrow mesenchymal stem cells is caused by reduced CCL2 expression. J Immunol. 193:5306–5314. DOI: 10.4049/jimmunol.1400036. PMID: 25339674.
Article14. Schena F, Gambini C, Gregorio A, Mosconi M, Reverberi D, Gattorno M, Casazza S, Uccelli A, Moretta L, Martini A, Traggiai E. 2010; Interferon-γ-dependent inhibition of B cell activation by bone marrow-derived mesenchymal stem cells in a murine model of systemic lupus erythematosus. Arthritis Rheum. 62:2776–2786. DOI: 10.1002/art.27560. PMID: 20496367.
Article15. Liu O, Xu J, Ding G, Liu D, Fan Z, Zhang C, Chen W, Ding Y, Tang Z, Wang S. 2013; Periodontal ligament stem cells regulate B lymphocyte function via programmed cell death protein 1. Stem Cells. 31:1371–1382. DOI: 10.1002/stem.1387. PMID: 23553748.
Article16. Tabera S, Pérez-Simón JA, Díez-Campelo M, Sánchez-Abarca LI, Blanco B, López A, Benito A, Ocio E, Sánchez-Guijo FM, Cañizo C, San Miguel JF. 2008; The effect of mesenchymal stem cells on the viability, proliferation and differentiation of B-lymphocytes. Haematologica. 93:1301–1309. DOI: 10.3324/haematol.12857. PMID: 18641017.
Article17. Khare D, Or R, Resnick I, Barkatz C, Almogi-Hazan O, Avni B. 2018; Mesenchymal stromal cell-derived exosomes affect mRNA expression and function of B-lymphocytes. Front Immunol. 9:3053. DOI: 10.3389/fimmu.2018.03053. PMID: 30622539. PMCID: PMC6308164.
Article18. Che N, Li X, Zhou S, Liu R, Shi D, Lu L, Sun L. 2012; Umbilical cord mesenchymal stem cells suppress B-cell proliferation and differentiation. Cell Immunol. 274:46–53. DOI: 10.1016/j.cellimm.2012.02.004. PMID: 22414555.
Article19. Krebs DL, Hilton DJ. 2001; SOCS proteins: negative regulators of cytokine signaling. Stem Cells. 19:378–387. DOI: 10.1634/stemcells.19-5-378. PMID: 11553846.
Article20. Yoshimura A, Suzuki M, Sakaguchi R, Hanada T, Yasukawa H. 2012; SOCS, inflammation, and autoimmunity. Front Immunol. 3:20. DOI: 10.3389/fimmu.2012.00020. PMID: 22566904. PMCID: PMC3342034.
Article21. Kubo M, Hanada T, Yoshimura A. 2003; Suppressors of cytokine signaling and immunity. Nat Immunol. 4:1169–1176. DOI: 10.1038/ni1012. PMID: 14639467.
Article22. Palmer DC, Restifo NP. 2009; Suppressors of cytokine signaling (SOCS) in T cell differentiation, maturation, and function. Trends Immunol. 30:592–602. DOI: 10.1016/j.it.2009.09.009. PMID: 19879803. PMCID: PMC2787651.
Article23. Li W, Ren G, Huang Y, Su J, Han Y, Li J, Chen X, Cao K, Chen Q, Shou P, Zhang L, Yuan ZR, Roberts AI, Shi S, Le AD, Shi Y. 2012; Mesenchymal stem cells: a double-edged sword in regulating immune responses. Cell Death Differ. 19:1505–1513. DOI: 10.1038/cdd.2012.26. PMID: 22421969. PMCID: PMC3422473.
Article24. Rodriguez LA 2nd, Mohammadipoor A, Alvarado L, Kamucheka RM, Asher AM, Cancio LC, Antebi B. 2019; Preconditioning in an inflammatory milieu augments the immunotherapeutic function of mesenchymal stromal cells. Cells. 8:E462. DOI: 10.3390/cells8050462. PMID: 31096722. PMCID: PMC6562603.
Article25. Ankrum JA, Ong JF, Karp JM. 2014; Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol. 32:252–260. DOI: 10.1038/nbt.2816. PMID: 24561556. PMCID: PMC4320647.
Article26. Zhang L, Dang RJ, Li H, Li P, Yang YM, Guo XM, Wang XY, Fang NZ, Mao N, Wen N, Jiang XX. 2014; SOCS1 regulates the immune modulatory properties of mesenchymal stem cells by inhibiting nitric oxide production. PLoS One. 9:e97256. DOI: 10.1371/journal.pone.0097256. PMID: 24826993. PMCID: PMC4020773.
Article27. Zhang L, Dang RJ, Yang YM, Cui DC, Li P, Ni YL, Hao T, Wang C, Jiang XX, Fang NZ. 2016; Delta-like-1 changes the immunomodulatory property of OP9 cells. Stem Cells Int. 2016:1628352. DOI: 10.1155/2016/1628352. PMID: 26649045. PMCID: PMC4663344.
Article28. Tezuka H, Abe Y, Iwata M, Takeuchi H, Ishikawa H, Matsushita M, Shiohara T, Akira S, Ohteki T. 2007; Regulation of IgA production by naturally occurring TNF/iNOS-producing dendritic cells. Nature. 448:929–933. DOI: 10.1038/nature06033. PMID: 17713535.
Article29. Brown DM, Phipps RP. 1995; Characterization and regulation of prostaglandin E2 receptors on normal and malignant murine B lymphocytes. Cell Immunol. 161:79–87. DOI: 10.1006/cimm.1995.1011. PMID: 7867087.
Article30. Renner P, Eggenhofer E, Rosenauer A, Popp FC, Steinmann JF, Slowik P, Geissler EK, Piso P, Schlitt HJ, Dahlke MH. 2009; Mesenchymal stem cells require a sufficient, ongoing immune response to exert their immunosuppressive function. Transplant Proc. 41:2607–2611. DOI: 10.1016/j.transproceed.2009.06.119. PMID: 19715984.
Article31. Najar M, Raicevic G, Boufker HI, Fayyad Kazan H, De Bruyn C, Meuleman N, Bron D, Toungouz M, Lagneaux L. 2010; Mesenchymal stromal cells use PGE2 to modulate activation and proliferation of lymphocyte subsets: combined comparison of adipose tissue, Wharton's Jelly and bone marrow sources. Cell Immunol. 264:171–179. DOI: 10.1016/j.cellimm.2010.06.006. PMID: 20619400.
Article32. Yañez R, Oviedo A, Aldea M, Bueren JA, Lamana ML. 2010; Prostaglandin E2 plays a key role in the immunosuppressive properties of adipose and bone marrow tissue-derived mesenchymal stromal cells. Exp Cell Res. 316:3109–3123. DOI: 10.1016/j.yexcr.2010.08.008. PMID: 20804749.
Article33. Garrone P, Galibert L, Rousset F, Fu SM, Banchereau J. 1994; Regulatory effects of prostaglandin E2 on the growth and differentiation of human B lymphocytes activated through their CD40 antigen. J Immunol. 152:4282–4290.34. Lee MR, Seo GY, Kim YM, Kim PH. 2011; iNOS potentiates mouse Ig isotype switching through AID expression. Biochem Biophys Res Commun. 410:602–607. DOI: 10.1016/j.bbrc.2011.06.035. PMID: 21684254.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Applications of Bioinspired Platforms for Enhancing Immunomodulatory Function of Mesenchymal Stromal Cells
- Immunomodulatory Effects of Placenta-derived Mesenchymal Stem Cells on T Cells by Regulation of FoxP3 Expression
- Suppression of miR-155 Expression in IFN-gamma-Treated Astrocytes and Microglia by DJ-1: A Possible Mechanism for Maintaining SOCS1 Expression
- A Novel Immunomodulatory Mechanism Dependent on Acetylcholine Secreted by Human Bone Marrow-derived Mesenchymal Stem Cells
- Mesenchymal Stromal Cells and Toll-Like Receptor Priming: A Critical Review