Korean Circ J.
2020 Jul;50(7):613-624. 10.4070/kcj.2019.0421.
Inhibition of Smooth Muscle Cell Proliferation and Migration by a Talin Modulator Attenuates Neointimal Formation after Femoral Arterial Injury
- Affiliations
-
- 1Department of Cardiology, Cardiovascular Center, Korea University College of Medicine, Seoul, Korea
- 2Department of Life Sciences, Korea University College of Life Sciences and Biotechnology, Seoul, Korea
- 3Research Institute of Pharmaceutical Sciences, College of Pharmacy, Kyungpook National University, Daegu, Korea
- KMID: 2504221
- DOI: http://doi.org/10.4070/kcj.2019.0421
Abstract
- Background and Objectives
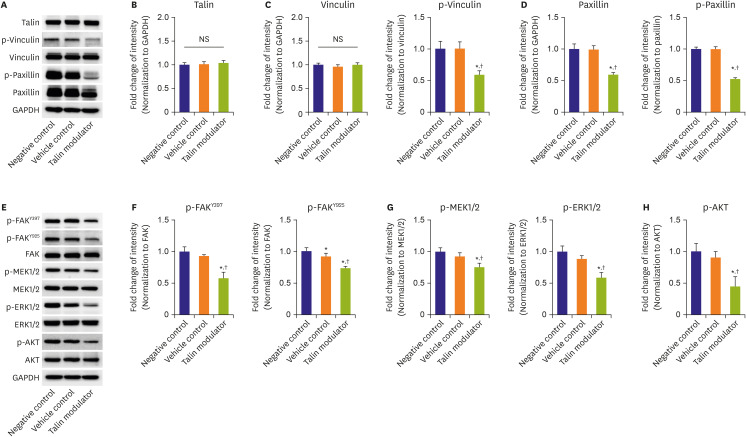
Vascular smooth muscle cell (SMC) proliferation and migration play a critical role in neointimal formation. Focal adhesion is involved in cell proliferation and migration, and talin is known to be a key regulator of these processes. We synthesized a new talin modulator that binds to the talin protein, and investigated its effects on SMCs and neointimal formation after vascular injury.
Methods
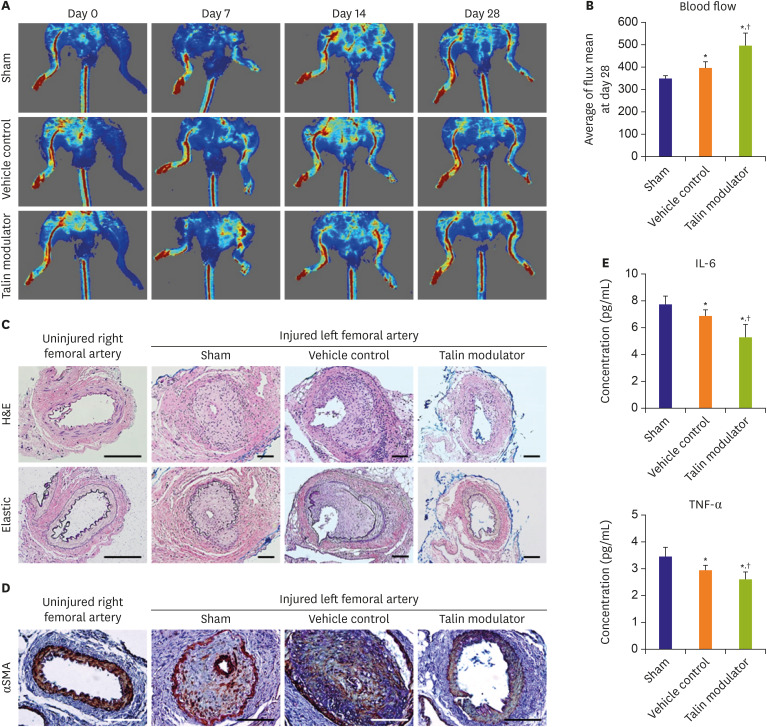
Human aortic SMCs (HAoSMCs) were treated with a newly synthesized talin modulator. Apolipoprotein E knockout (ApoE KO) mice were subjected to left femoral arterial injury and orally administered with the talin modulator daily. Laser Doppler imager was used to compare the blood flow, and injured femoral arteries and blood serum were analyzed after 28 days.
Results
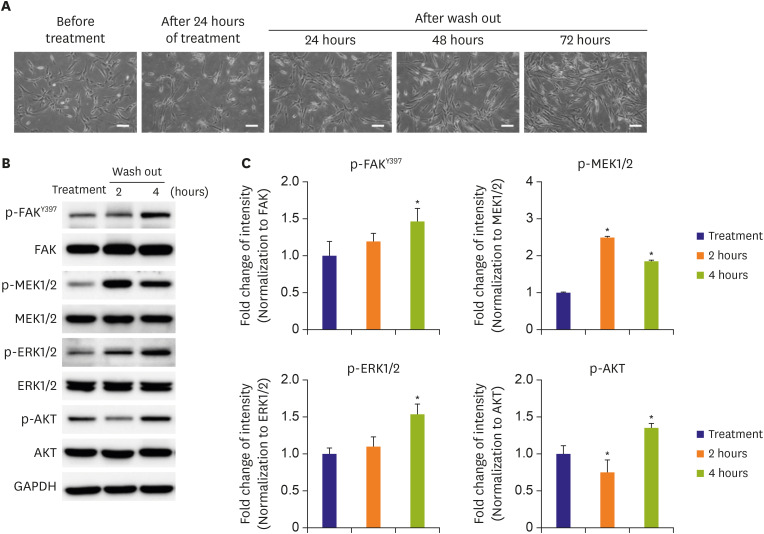
The talin modulator significantly inhibited cell proliferation in a concentration-dependent manner and suppressed the migration of HAoSMCs. Treatment with a talin modulator resulted in a significant reduction in the phosphorylation of focal adhesion molecules and downstream signaling molecules related to cell proliferation and migration. The effects of the talin modulator in HAoSMCs were found to be reversible, as evidenced by the reactivation of signaling pathways upon its removal. After 28 days of administration of the talin modulator, an improvement in the blood flow and reduction in neointimal formation in the injured femoral arteries were observed.
Conclusions
We demonstrated the inhibitory effects of a talin modulator on SMC proliferation and migration, and that were associated with downregulation of signaling pathways, resulting in the attenuation of neointimal formation in ApoE KO mice.
Figure
Cited by 1 articles
-
The New Weapon to Inhibit Proliferation and Migration of Smooth Muscle Cell in Neointimal Formation
Cheong-Whan Chae, Yoo-Wook Kwon
Korean Circ J. 2020;50(7):625-627. doi: 10.4070/kcj.2020.0197.
Reference
-
1. Bennett MR, Sinha S, Owens GK. Vascular smooth muscle cells in atherosclerosis. Circ Res. 2016; 118:692–702. PMID: 26892967.
Article2. Kim JH, Lim IR, Joo HJ, et al. Fimasartan reduces neointimal formation and inflammation after carotid arterial injury in apolipoprotein E knockout mice. Mol Med. 2019; 25:33. PMID: 31307370.
Article3. Nagayama K, Kyotani Y, Zhao J, et al. Exendin-4 prevents vascular smooth muscle cell proliferation and migration by angiotensin II via the inhibition of ERK1/2 and JNK signaling pathways. PLoS One. 2015; 10:e0137960. PMID: 26379274.
Article4. Huttenlocher A, Horwitz AR. Integrins in cell migration. Cold Spring Harb Perspect Biol. 2011; 3:a005074. PMID: 21885598.
Article5. Wozniak MA, Modzelewska K, Kwong L, Keely PJ. Focal adhesion regulation of cell behavior. Biochim Biophys Acta. 2004; 1692:103–119. PMID: 15246682.
Article6. Sun Z, Costell M, Fässler R. Integrin activation by talin, kindlin and mechanical forces. Nat Cell Biol. 2019; 21:25–31. PMID: 30602766.
Article7. Ye F, Kim C, Ginsberg MH. Reconstruction of integrin activation. Blood. 2012; 119:26–33. PMID: 21921044.
Article8. Goult BT, Yan J, Schwartz MA. Talin as a mechanosensitive signaling hub. J Cell Biol. 2018; 217:3776–3784. PMID: 30254032.
Article9. Wang P, Ballestrem C, Streuli CH. The C terminus of talin links integrins to cell cycle progression. J Cell Biol. 2011; 195:499–513. PMID: 22042621.
Article10. Harburger DS, Calderwood DA. Integrin signalling at a glance. J Cell Sci. 2009; 122:159–163. PMID: 19118207.
Article11. Chen HC, Appeddu PA, Parsons JT, Hildebrand JD, Schaller MD, Guan JL. Interaction of focal adhesion kinase with cytoskeletal protein talin. J Biol Chem. 1995; 270:16995–16999. PMID: 7622520.
Article12. Calalb MB, Polte TR, Hanks SK. Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: a role for Src family kinases. Mol Cell Biol. 1995; 15:954–963. PMID: 7529876.
Article13. Kleinschmidt EG, Schlaepfer DD. Focal adhesion kinase signaling in unexpected places. Curr Opin Cell Biol. 2017; 45:24–30. PMID: 28213315.
Article14. Bouchard V, Demers MJ, Thibodeau S, et al. Fak/Src signaling in human intestinal epithelial cell survival and anoikis: differentiation state-specific uncoupling with the PI3-K/Akt-1 and MEK/Erk pathways. J Cell Physiol. 2007; 212:717–728. PMID: 17443665.
Article15. Tomakidi P, Schulz S, Proksch S, Weber W, Steinberg T. Focal adhesion kinase (FAK) perspectives in mechanobiology: implications for cell behaviour. Cell Tissue Res. 2014; 357:515–526. PMID: 24988914.
Article16. Lim IR, Joo HJ, Jeong M, et al. Talin modulation by a synthetic N-acylurea derivative reduces angiogenesis in human endothelial cells. Int J Mol Sci. 2017; 18:E221. PMID: 28117756.
Article17. Kaneda T, Sonoda Y, Ando K, et al. Mutation of Y925F in focal adhesion kinase (FAK) suppresses melanoma cell proliferation and metastasis. Cancer Lett. 2008; 270:354–361. PMID: 18606490.
Article18. Xia H, Nho RS, Kahm J, Kleidon J, Henke CA. Focal adhesion kinase is upstream of phosphatidylinositol 3-kinase/Akt in regulating fibroblast survival in response to contraction of type I collagen matrices via a beta 1 integrin viability signaling pathway. J Biol Chem. 2004; 279:33024–33034. PMID: 15166238.19. Kim J, Jang SW, Park E, Oh M, Park S, Ko J. The role of heat shock protein 90 in migration and proliferation of vascular smooth muscle cells in the development of atherosclerosis. J Mol Cell Cardiol. 2014; 72:157–167. PMID: 24650873.
Article20. Daniel JM, Prock A, Dutzmann J, et al. Regulator of G-protein signaling 5 prevents smooth muscle cell proliferation and attenuates neointima formation. Arterioscler Thromb Vasc Biol. 2016; 36:317–327. PMID: 26663397.
Article21. Son JE, Lee E, Jung SK, et al. Anthocyanidins, novel FAK inhibitors, attenuate PDGF-BB-induced aortic smooth muscle cell migration and neointima formation. Cardiovasc Res. 2014; 101:503–512. PMID: 24363205.
Article22. Katsuda S, Okada Y, Nakanishi I, Tanaka J. Inhibitory effect of dimethyl sulfoxide on the proliferation of cultured arterial smooth muscle cells: relationship to the cytoplasmic microtubules. Exp Mol Pathol. 1988; 48:48–58. PMID: 3335251.
Article23. Camici GG, Steffel J, Akhmedov A, et al. Dimethyl sulfoxide inhibits tissue factor expression, thrombus formation, and vascular smooth muscle cell activation: a potential treatment strategy for drug-eluting stents. Circulation. 2006; 114:1512–1521. PMID: 17000906.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Perivascular Delivery of Paclitaxel with F-127 Pluronic Gel Inhibits Neointimal Hyperplasia in a Rat Carotid Artery Injury Model
- Effect of Udenafil on Vascular Smooth Muscle Cell Proliferation and Neointimal Hyperplasia in Rat Carotid Artery Injury Model
- Inhibition of neointimal proliferation of rat carotid artery by sulodexide
- Intimal Hyperplasia in Vascular Grafts: Surgery-induced Arteriosclerosis
- Restenosis and Remodeling