Korean J Transplant.
2020 Mar;34(1):15-23. 10.4285/kjt.2020.34.1.15.
Effectiveness of valacyclovir prophylaxis against the occurrence of cytomegalovirus infection in kidney transplant recipients
- Affiliations
-
- 1Department of Internal Medicine, Keimyung University School of Medicine, Daegu, Korea
- 2Keimyung University Kidney Institute, Daegu, Korea
- KMID: 2503778
- DOI: http://doi.org/10.4285/kjt.2020.34.1.15
Abstract
- Background
Cytomegalovirus (CMV) infection is a crucial infection in kidney transplant recipients (KTRs) despite advancements in diagnostic and treatment methods. There are still many controversies about the ways to prevent CMV infection.
Methods
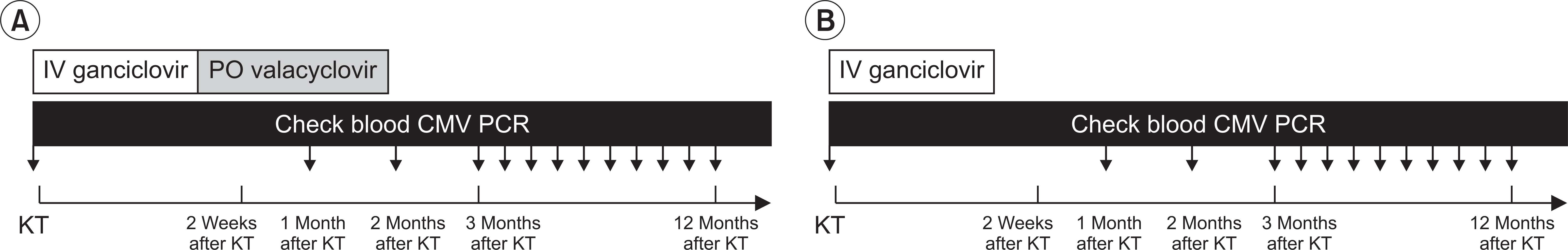
We retrospectively analyzed 153 KTRs who underwent kidney transplantation (KT) between September 2013 and January 2016. We classified KTRs into two groups: valacyclovir prophylaxis group (intravenous ganciclovir for 2 weeks after KT, followed by oral valacyclovir for 3 months) and historical control group (only intravenous ganciclovir for 2 weeks after KT). We evaluated the incidence of CMV infection, clinical outcomes, CMV-free survival rate between the two groups and risk factors for the development of CMV infection.
Results
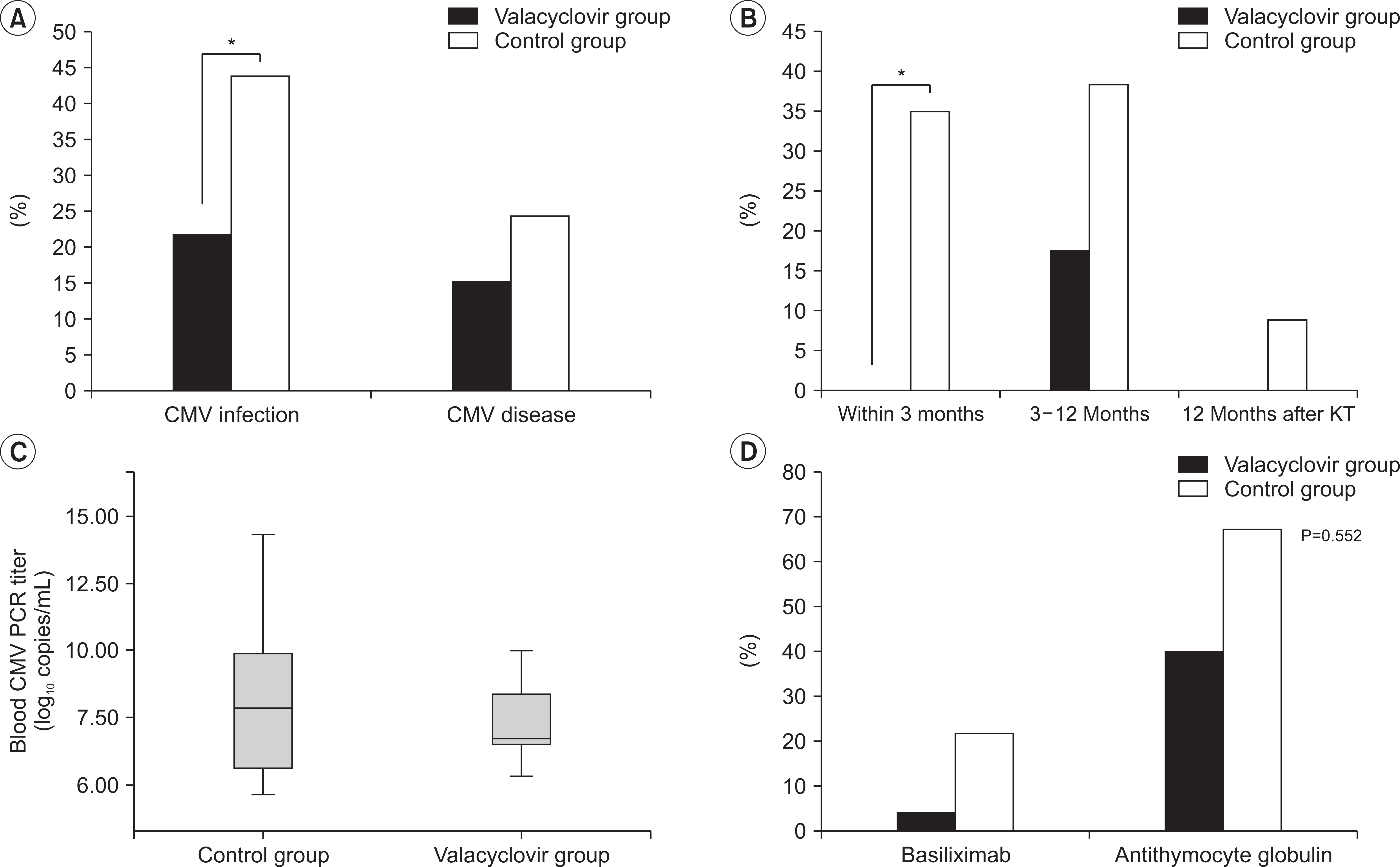
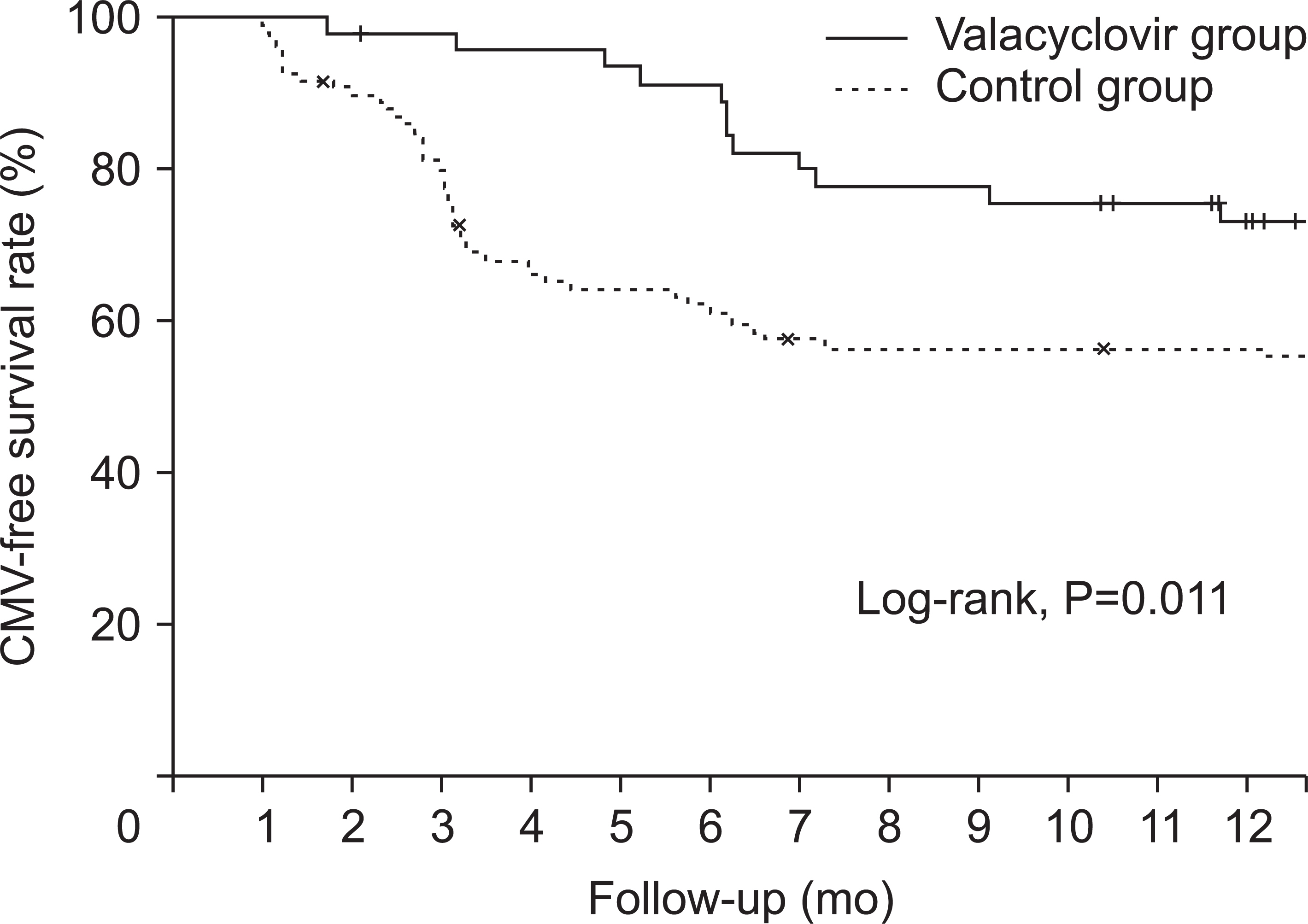
Mean time between KT and diagnosis of CMV infection was 4.5±3.3 months. The valacyclovir prophylaxis group showed lower incidence of CMV infection than the historical control group (21.7% vs. 43.9%, P=0.011). The valacyclovir prophylaxis group showed higher CMV-free survival rate than the control group (P=0.011). In multivariable- adjusted analysis, independent risk factors for the development of CMV infection were no valacyclovir prophylaxis, older age at KT, thymoglobulin induction, and delayed graft function.
Conclusions
Valacyclovir prophylaxis for 3 months showed significant reduction in the incidence of CMV infection in KTRs. Therefore, we suggest valacyclovir prophylaxis for 3 months in KTRs with risk factors such as old age, thymoglobulin induction, and delayed graft function.
Keyword
Figure
Reference
-
Fishman JA. 2007. Infection in solid-organ transplant recipients. N Engl J Med. 357:2601–14. DOI: 10.1056/NEJMra064928. PMID: 18094380.
ArticleHill P., Cross NB., Barnett AN., Palmer SC., Webster AC. 2017. Polyclonal and monoclonal antibodies for induction therapy in kidney transplant recipients. Cochrane Database Syst Rev. 1:CD004759. DOI: 10.1002/14651858.CD004759.pub2. PMCID: PMC6464766. PMID: 28073178.
ArticleDe Keyzer K., Van Laecke S., Peeters P., Vanholder R. 2011. Human cytomegalovirus and kidney transplantation: a clinician's update. Am J Kidney Dis. 58:118–26. DOI: 10.1053/j.ajkd.2011.04.010. PMID: 21684438.
ArticleFreeman RB Jr. 2009. The 'indirect' effects of cytomegalovirus infection. Am J Transplant. 9:2453–8. DOI: 10.1111/j.1600-6143.2009.02824.x. PMID: 19843027.Sagedal S., Nordal KP., Hartmann A., Sund S., Scott H., Degré M, et al. 2002. The impact of cytomegalovirus infection and disease on rejection episodes in renal allograft recipients. Am J Transplant. 2:850–6. DOI: 10.1034/j.1600-6143.2002.20907.x. PMID: 12392291.
ArticleRazonable RR., Humar A. AST Infectious Diseases Community of Practice. 2013. Cytomegalovirus in solid organ transplantation. Am J Transplant. 13(Suppl 4):93–106. DOI: 10.1111/ajt.12103. PMID: 23465003.
ArticleKidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. 2009. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 9(Suppl 3):S1–155. DOI: 10.1111/j.1600-6143.2009.02834.x. PMID: 19845597.Kotton CN., Kumar D., Caliendo AM., Asberg A., Chou S., Danziger-Isakov L, et al. 2013. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 96:333–60. DOI: 10.1097/TP.0b013e31829df29d. PMID: 23896556.
ArticleReischig T., Kacer M., Jindra P., Hes O., Lysak D., Bouda M. 2015. Randomized trial of valganciclovir versus valacyclovir prophylaxis for prevention of cytomegalovirus in renal transplantation. Clin J Am Soc Nephrol. 10:294–304. DOI: 10.2215/CJN.07020714. PMID: 25424991. PMCID: PMC4317746.
ArticleLjungman P., Boeckh M., Hirsch HH., Josephson F., Lundgren J., Nichols G, et al. 2017. Definitions of cytomegalovirus infection and disease in transplant patients for use in clinical trials. Clin Infect Dis. 64:87–91. DOI: 10.1093/cid/ciw668. PMID: 27682069.Lowance D., Neumayer HH., Legendre CM., Squifflet JP., Kovarik J., Brennan PJ, et al. 1999. Valacyclovir for the prevention of cytomegalovirus disease after renal transplantation. International Valacyclovir Cytomegalovirus Prophylaxis Transplantation Study Group. N Engl J Med. 340:1462–70. DOI: 10.1056/NEJM199905133401903. PMID: 10320384.Paya C., Humar A., Dominguez E., Washburn K., Blumberg E., Alexander B, et al. 2004. Efficacy and safety of valganciclovir vs. oral ganciclovir for prevention of cytomegalovirus disease in solid organ transplant recipients. Am J Transplant. 4:611–20. DOI: 10.1111/j.1600-6143.2004.00382.x. PMID: 15023154.
ArticleKacer M., Kielberger L., Bouda M., Reischig T. 2015. Valganciclovir versus valacyclovir prophylaxis for prevention of cytomegalovirus: an economic perspective. Transpl Infect Dis. 17:334–41. DOI: 10.1111/tid.12383. PMID: 25824586.
ArticlePuttarajappa C., Bhattarai M., Mour G., Shen C., Sood P., Mehta R, et al. 2016. Cytomegalovirus infection in high-risk kidney transplant recipients receiving thymoglobulin induction-a single-center experience. Clin Transplant. 30:1159–64. DOI: 10.1111/ctr.12810. PMID: 27423138.
ArticleElfadawy N., Flechner SM., Liu X., Schold J., Srinivas TR., Poggio E, et al. 2013. CMV Viremia is associated with a decreased incidence of BKV reactivation after kidney and kidney-pancreas transplantation. Transplantation. 96:1097–103. DOI: 10.1097/TP.0b013e3182a6890d. PMID: 24056621.
ArticleSund F., Tufveson G., Döhler B., Opelz G., Eriksson BM. 2013. Clinical outcome with low-dose valacyclovir in high-risk renal transplant recipients: a 10-year experience. Nephrol Dial Transplant. 28:758–65. DOI: 10.1093/ndt/gfs531. PMID: 23243043.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Usefulness of valacyclovir prophylaxis for cytomegalovirus infection after anti-thymocyte globulin as rejection therapy
- CMV Disease
- Current trends and clinical impact of cytomegalovirus prophylaxis in kidney transplant recipients in Korea: the Korean Organ Transplantation Registry study
- Human Cytomegalovirus Infection in Solid-Organ Transplantation
- Disseminated Cytomegalovirus Infection after Renal Transplantation: A Case Report