Korean J Transplant.
2020 Mar;34(1):8-14. 10.4285/kjt.2020.34.1.8.
Comparative evaluation of QuantiFERON-TB Gold Plus for diagnosis of latent tuberculosis infection during solid organ transplantation
- Affiliations
-
- 1Department of Laboratory Medicine and Genetics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 2Division of Nephrology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 3Department of Transplantation Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 4Division of Gastroenterology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 5Division of Cardiology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2503777
- DOI: http://doi.org/10.4285/kjt.2020.34.1.8
Abstract
- Background
The diagnosis of latent tuberculosis infection (LTBI) in solid organ transplantation (SOT) patients under lifelong immunosuppression has profound effects on preoperative and postoperative management. Interferon-gamma release assay (IGRA) is widely used to screen LTBI before or after transplantation.
Methods
We evaluated the effect of posttransplantation immunosuppression on IGRA and influencing factors by measuring interval change of QuantiFERON-TB Gold Plus (QFT-Plus) between pretransplantation (pre-QFT-Plus) and posttransplantation (post-QFT-Plus) state in 20 patients who previously had reactive IGRA but not taken LTBI treatment.
Results
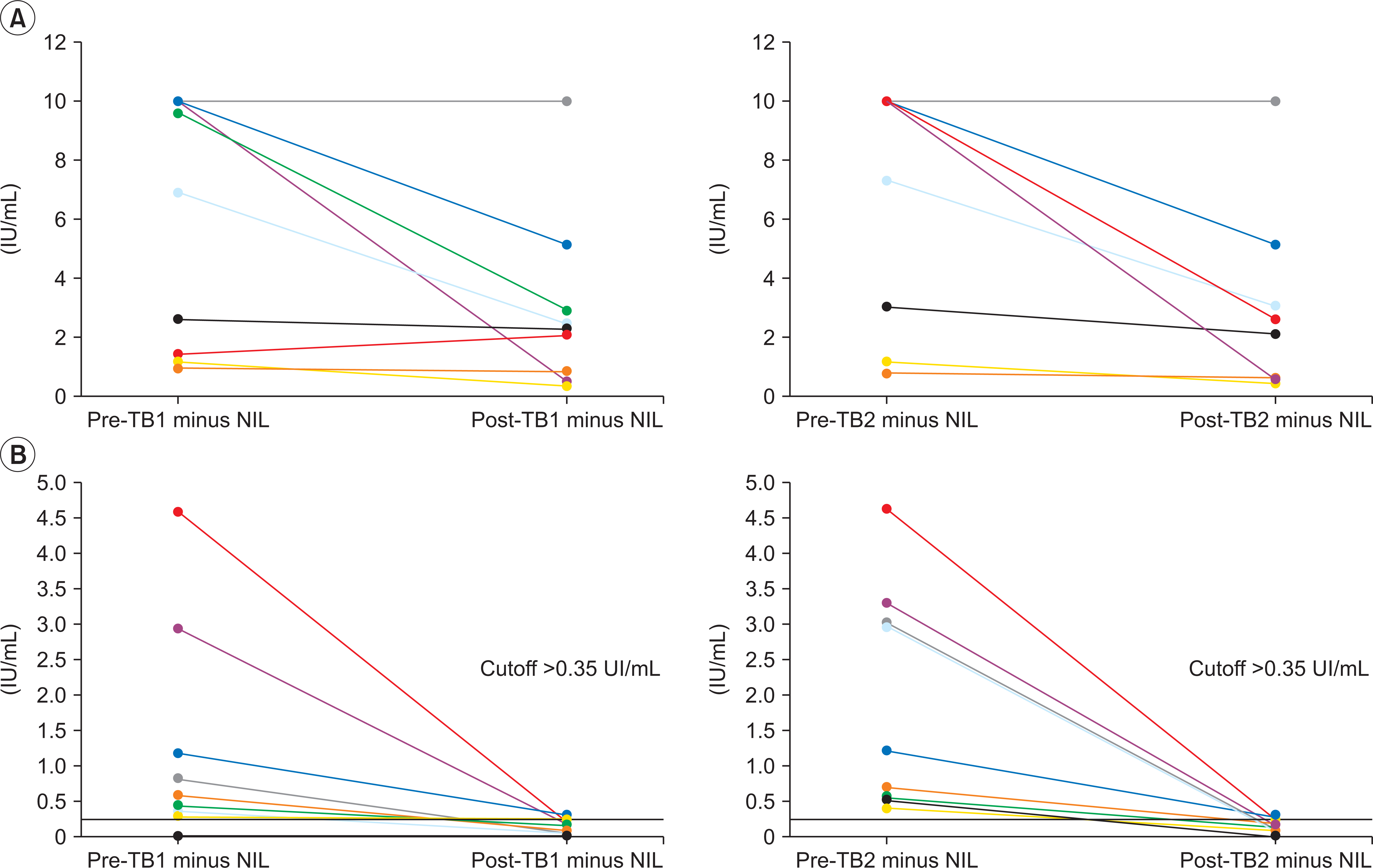
Eleven (55%) out of 20 pre-QFT-Plus reactive patients became nonreactive state in repeated QFT-Plus (post-QFT-Plus) at 194–413 days (median, 257 days) after transplantation (discordant group). Even in persistently reactive group (concordant group), interferon-gamma (IFN-γ) levels after transplantation were decreased about 34% and 36% of their pretransplantation levels for TB1 and TB2, respectively. The only significant factors that affect interval change of QFT-Plus between pre- and post-SOT status were the concentrations of IFN-γ in pre-QFT-Plus (6.93 vs. 0.44 IU/mL in TB1 and 7.33 vs. 0.71 IU/mL in TB2).
Conclusions
The reactivity of QFT-Plus is significantly compromised by immunosuppressive therapy, which increase the risk of false negative, particularly in patients with low level of IGRA reactivity. Therefore, interpretation of IGRA under immunosuppressive treatment require a caution and eventually, more sensitive tuberculosis-specific cytokine markers might be needed.
Figure
Reference
-
Dheda K., Barry CE 3rd., Maartens G. 2016. Tuberculosis. Lancet. 387:1211–26. DOI: 10.1016/S0140-6736(15)00151-8. PMID: 26377143.
ArticleAguado JM., Herrero JA., Gavaldá J., Torre-Cisneros J., Blanes M., Rufí G, et al. 1997. Clinical presentation and outcome of tuberculosis in kidney, liver, and heart transplant recipients in Spain. Spanish Transplantation Infection Study Group, GESITRA. Transplantation. 63:1278–86. DOI: 10.1097/00007890-199705150-00015. PMID: 9158022.Singh N., Paterson DL. 1998. Mycobacterium tuberculosis infection in solid-organ transplant recipients: impact and implications for management. Clin Infect Dis. 27:1266–77. DOI: 10.1086/514993. PMID: 9827281.Bumbacea D., Arend SM., Eyuboglu F., Fishman JA., Goletti D., Ison MG, et al. 2012. The risk of tuberculosis in transplant candidates and recipients: a TBNET consensus statement. Eur Respir J. 40:990–1013. DOI: 10.1183/09031936.00000712. PMID: 22496318.
ArticlePai M., Behr MA., Dowdy D., Dheda K., Divangahi M., Boehme CC, et al. 2016. Tuberculosis. Nat Rev Dis Primers. 2:16076. DOI: 10.1038/nrdp.2016.76. PMID: 27784885.
ArticleAuguste P., Tsertsvadze A., Pink J., Court R., McCarthy N., Sutcliffe P, et al. 2017. Comparing interferon-gamma release assays with tuberculin skin test for identifying latent tuberculosis infection that progresses to active tuberculosis: systematic review and meta-analysis. BMC Infect Dis. 17:200. DOI: 10.1186/s12879-017-2301-4. PMID: 28274215. PMCID: PMC5343308.
ArticleKim EY., Lim JE., Jung JY., Son JY., Lee KJ., Yoon YW, et al. 2009. Performance of the tuberculin skin test and interferon-gamma release assay for detection of tuberculosis infection in immunocompromised patients in a BCG-vaccinated population. BMC Infect Dis. 9:207. DOI: 10.1186/1471-2334-9-207. PMID: 20003535. PMCID: PMC2801508.
ArticleSester M., van Leth F., Bruchfeld J., Bumbacea D., Cirillo DM., Dilektasli AG, et al. 2014. Risk assessment of tuberculosis in immunocompromised patients. A TBNET study. Am J Respir Crit Care Med. 190:1168–76. DOI: 10.1164/rccm.201405-0967OC. PMID: 25303140.
ArticleEkberg H., Tedesco-Silva H., Demirbas A., Vítko S., Nashan B., Gürkan A, et al. 2007. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med. 357:2562–75. DOI: 10.1056/NEJMoa067411. PMID: 18094377.
ArticleWatson CJ., Dark JH. 2012. Organ transplantation: historical perspective and current practice. Br J Anaesth. 108(Suppl 1):i29–42. DOI: 10.1093/bja/aer384. PMID: 22194428.
ArticleCollett D., Mumford L., Banner NR., Neuberger J., Watson C. 2010. Comparison of the incidence of malignancy in recipients of different types of organ: a UK Registry audit. Am J Transplant. 10:1889–96. DOI: 10.1111/j.1600-6143.2010.03181.x. PMID: 20659094.
ArticleLee SH. 2018. Diagnosis and treatment of latent tuberculosis infection: the updated 2017 Korean guidelines. Korean J Med. 93:509–17. DOI: 10.3904/kjm.2018.93.6.509.
ArticleRyu MR., Park MS., Cho EH., Jung CW., Kim K., Kim SJ, et al. 2018. Comparative evaluation of QuantiFERON-TB Gold In-Tube and QuantiFERON-TB Gold Plus in diagnosis of latent tuberculosis infection in immunocompromised patients. J Clin Microbiol. 56:e00438–18. DOI: 10.1128/JCM.00438-18. PMID: 30135226. PMCID: PMC6204680.
ArticleTheel ES., Hilgart H., Breen-Lyles M., McCoy K., Flury R., Breeher LE, et al. 2018. Comparison of the QuantiFERON-TB Gold Plus and QuantiFERON-TB Gold In-Tube interferon gamma release assays in patients at risk for tuberculosis and in health care workers. J Clin Microbiol. 56:e00614–18. DOI: 10.1128/JCM.00614-18. PMID: 29743310. PMCID: PMC6018330.
ArticleYi L., Sasaki Y., Nagai H., Ishikawa S., Takamori M., Sakashita K, et al. 2016. Evaluation of QuantiFERON-TB Gold Plus for detection of Mycobacterium tuberculosis infection in Japan. Sci Rep. 6:30617. DOI: 10.1038/srep30617. PMID: 27470684. PMCID: PMC4965764.
ArticleDe Groote MA., Higgins M., Hraha T., Wall K., Wilson ML., Sterling DG, et al. 2017. Highly multiplexed proteomic analysis of quantiferon supernatants to identify biomarkers of latent tuberculosis infection. J Clin Microbiol. 55:391–402. DOI: 10.1128/JCM.01646-16. PMID: 27852671. PMCID: PMC5277508.
ArticleGunluoglu G., Seyhan EC., Kazancioglu R., Gunluoglu Z., Veske NS., Yazar EE, et al. 2015. Diagnosing latent tuberculosis in immunocompromised patients measuring blood IP-10 production capacity: an analysis of chronic renal failure patients. Intern Med. 54:465–72. DOI: 10.2169/internalmedicine.54.3245. PMID: 25758071.
ArticleJenum S., Dhanasekaran S., Ritz C., Macaden R., Doherty TM., Grewal HM, et al. 2016. Added value of IP-10 as a read-out of mycobacterium tuberculosis: specific immunity in young children. Pediatr Infect Dis J. 35:1336–8. DOI: 10.1097/INF.0000000000001328. PMID: 27642776. PMCID: PMC5108305.Wergeland I., Assmus J., Dyrhol-Riise AM. 2016. Cytokine patterns in tuberculosis infection; IL-1ra, IL-2 and IP-10 differentiate borderline QuantiFERON-TB samples from uninfected controls. PLoS One. 11:e0163848. DOI: 10.1371/journal.pone.0163848. PMID: 27685462. PMCID: PMC5042373.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Application of the QuantiFERON(R)-TB Gold Test in Two Cases of Erythema Induratum of Bazin
- Mycobacterial infections in solid organ transplant recipients
- Comparison of Interferon-gamma Assays with the Tuberculin Skin Test in Children
- The Diagnostic Value of Interferon-gamma Assay in Patients with Active Tuberculosis
- Tuberculin Skin Test and QuantiFERON-TB Gold Assay before and after Treatment for Latent Tuberculosis Infection among Health Care Workers in Local Tertiary Hospital