Blood Res.
2020 Jun;55(2):91-98. 10.5045/br.2020.2020031.
Natural soluble human leukocyte antigen class I in donor serum neutralizes donor-specific HLA alloantibodies in recipient serum
- Affiliations
-
- 1Departments of Clinical Pathology, School of Medicine, Kyungpook National University, Daegu, Korea
- 2Departments of Internal Medicine, School of Medicine, Kyungpook National University, Daegu, Korea
- 3Departments of Surgery, School of Medicine, Kyungpook National University, Daegu, Korea
- KMID: 2503483
- DOI: http://doi.org/10.5045/br.2020.2020031
Abstract
- Background
Human leukocyte antigen (HLA) molecules are cell-bound but can be identified in a soluble form. These soluble HLA (sHLA) molecules have an immunomodulatory function. We investigated whether natural sHLA in donor serum can neutralize donor-specific HLA alloantibodies (DSAs) in recipient serum.
Methods
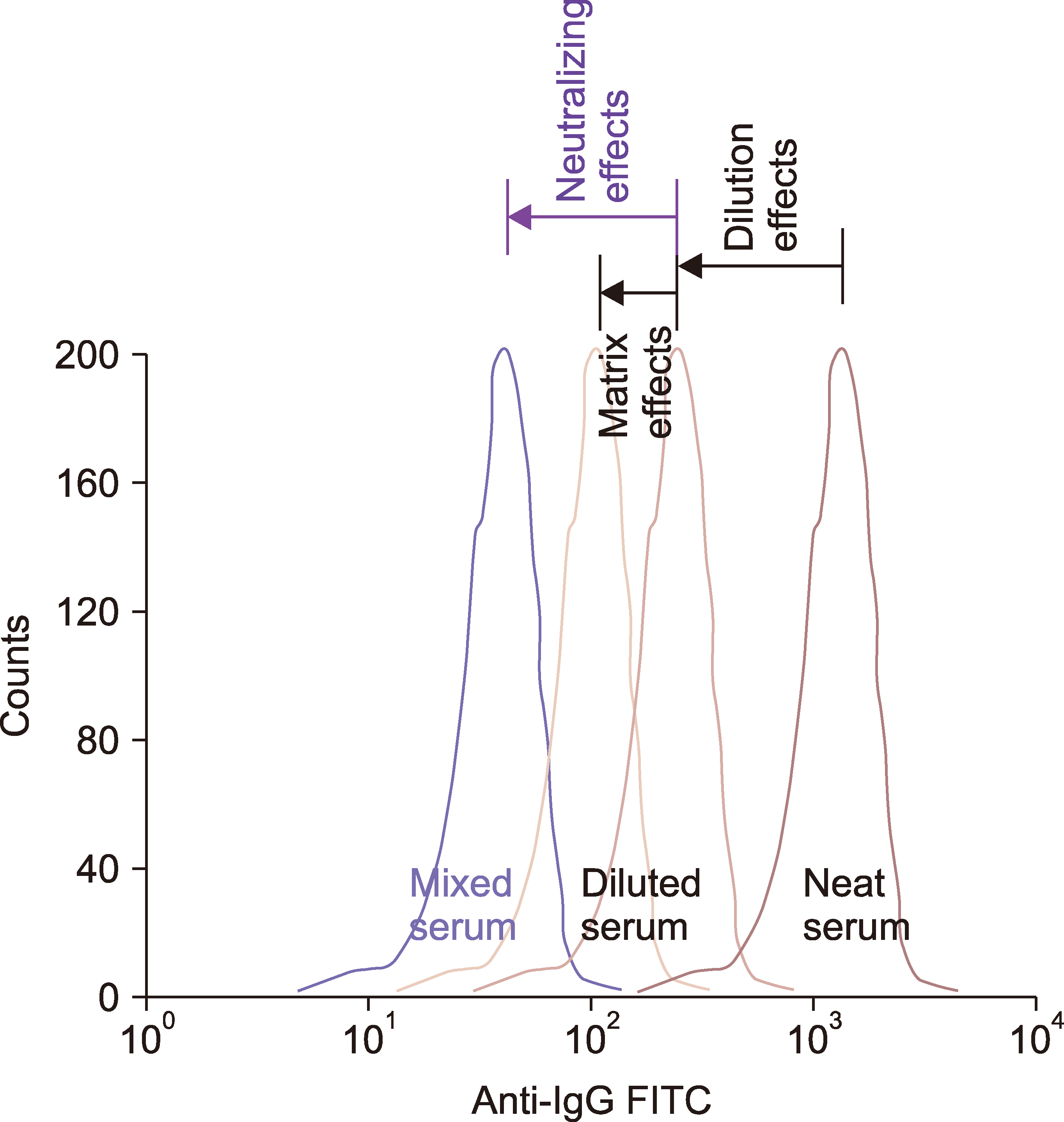
Neutralizing effects of donor serum on DSAs in recipient serum were measured using inhibition assay principle of flow cytometric crossmatch (FCXM), performed using sera from 143 kidney transplant recipients and their donors. The adding of donor serum to recipient serum yielded lower mean fluorescence intensity (MFI) ratios (test/control) than when diluent was added [Roswell Park Memorial Institute (RPMI) or third-party serum], which was presumed to be caused by the neutralizing effects of sHLA.
Results
In the recipient group with class I DSAs alone (N=14), donor serum addition to recipient serum resulted in lower T cell MFI ratios [2.25 (1.31‒32.51)] than those observed on RPMI addition [3.04 (1.33‒125.39), P <0.05]. In the recipient group with class II DSAs alone (N=27), donor serum addition showed no significant difference in B cell MFI ratios [5.03 (1.41‒103.53)] compared to diluent addition: RPMI [4.50 (1.34‒145.98)] or third-party serum [5.08 (1.44‒138.47)], P >0.05 for both.
Conclusion
Using inhibition FCXM, we verified that natural sHLA class I in donor serum neutralizes DSAs in recipient serum. However, no neutralizing effects of sHLA class II were revealed in this study. These potentially beneficial effects of sHLA infused via blood-derived products should be considered when desensitizing highly HLA-sensitized patients.
Figure
Reference
-
1. Adamashvili I, Kelley RE, Pressly T, McDonald JC. 2005; Soluble HLA: patterns of expression in normal subjects, autoimmune diseases, and transplant recipients. Rheumatol Int. 25:491–500. DOI: 10.1007/s00296-005-0585-y. PMID: 15986087.
Article2. den Dulk M, Bishop GA. 2003; Immune mechanisms contributing to spontaneous acceptance of liver transplants in rodents and their potential for clinical transplantation. Arch Immunol Ther Exp (Warsz). 51:29–44. PMID: 12691302.3. Westhoff U, Grosse-Wilde H. 1995; Soluble HLA class I and class II concentrations in factor VIII and PCC preparations. Vox Sang. 68:73–6. DOI: 10.1159/000462899. PMID: 7762224.
Article4. McDonald JC, Gelder FB, Aultman DF, et al. 1992; HLA in human serum--quantitation of class I by enzyme immunoassay. Transplantation. 53:445–9. DOI: 10.1097/00007890-199202010-00034. PMID: 1738939.5. Takanashi M, Atsuta Y, Fujiwara K, et al. 2010; The impact of anti-HLA antibodies on unrelated cord blood transplantations. Blood. 116:2839–46. DOI: 10.1182/blood-2009-10-249219. PMID: 20628152.
Article6. Spellman S, Bray R, Rosen-Bronson S, et al. 2010; The detection of donor-directed, HLA-specific alloantibodies in recipients of unrelated hematopoietic cell transplantation is predictive of graft failure. Blood. 115:2704–8. DOI: 10.1182/blood-2009-09-244525. PMID: 20089963. PMCID: PMC2852369.
Article7. van Rood JJ, van Leeuwen A, van Santen MC. 1970; Anti HL-A2 inhibitor in normal human serum. Nature. 226:366–7. DOI: 10.1038/226366a0. PMID: 5309633.
Article8. Sumitran-Karuppan S, Möller E. 1994; Specific inhibition of HLA class I and II antibodies by soluble antigens--a method for the identification of antibody specificity in sera from alloimmunized individuals. Transplantation. 58:713–9. DOI: 10.1097/00007890-199409000-00013. PMID: 7940692.
Article9. Ghio M, Contini P, Mazzei C, et al. 1999; Soluble HLA class I, HLA class II, and Fas ligand in blood components: a possible key to explain the immunomodulatory effects of allogeneic blood transfusions. Blood. 93:1770–7. DOI: 10.1182/blood.V93.5.1770. PMID: 10029607.
Article10. Jordan SC, Vo AA, Peng A, Toyoda M, Tyan D. 2006; Intravenous gammaglobulin (IVIG): a novel approach to improve transplant rates and outcomes in highly HLA-sensitized patients. Am J Transplant. 6:459–66. DOI: 10.1111/j.1600-6143.2005.01214.x. PMID: 16468954.
Article11. Zachary AA, Montgomery RA, Leffell MS. 2005; Factors associated with and predictive of persistence of donor-specific antibody after treatment with plasmapheresis and intravenous immunoglobulin. Hum Immunol. 66:364–70. DOI: 10.1016/j.humimm.2005.01.032. PMID: 15866699.
Article12. Couzi L, Araujo C, Guidicelli G, et al. 2011; Interpretation of positive flow cytometric crossmatch in the era of the single-antigen bead assay. Transplantation. 91:527–35. DOI: 10.1097/TP.0b013e31820794bb. PMID: 21192319.
Article13. Grundbacher FJ, Shreffler DC. 1970; Effects of secretor, blood, and serum groups on isoantibody and immunoglobulin levels. Am J Hum Genet. 22:194–202. PMID: 4985153. PMCID: PMC1706527.14. Hasek M, Chutna J, Holan V, Sladecek M. 1976; Induction of transplantation tolerance using serum as antigen source. Nature. 262:295–6. DOI: 10.1038/262295a0. PMID: 785269.
Article15. Kamada N, Shinomiya T. 1985; Clonal deletion as the mechanism of abrogation of immunological memory following liver grafting in rats. Immunology. 55:85–90.16. Smith MA, Naziruddin B, Poindexter NJ, Haynes AE, Howard T, Mohanakumar T. 2000; Liver transplant recipient sera derived soluble HLA mediates allele specific CTL apoptosis. Transplantation. 69:157–62. DOI: 10.1097/00007890-200001150-00026. PMID: 10653395.
Article17. Creput C, Le Friec G, Bahri R, et al. 2003; Detection of HLA-G in serum and graft biopsy associated with fewer acute rejections following combined liver-kidney transplantation: possible implications for monitoring patients. Hum Immunol. 64:1033–8. DOI: 10.1016/j.humimm.2003.08.356. PMID: 14602232.
Article18. Ghio M, Contini P, Ubezio G, Mazzei C, Puppo F, Indiveri F. 2008; Immunomodulatory effects of blood transfusions: the synergic role of soluble HLA class I free heavy-chain molecules detectable in blood components. Transfusion. 48:1591–7. DOI: 10.1111/j.1537-2995.2008.01720.x. PMID: 18466172.19. Guencheva G, Scholz S, Schiessl B, Albert ED. 1982; Soluble HLA antigens in normal human immunoglobulin preparations. Tissue Antigens. 19:198–204. DOI: 10.1111/j.1399-0039.1982.tb01440.x. PMID: 6178182.
Article20. Xu H, Montgomery SP, Preston EH, et al. 2003; Studies investigating pretransplant donor-specific blood transfusion, rapamycin, and the CD154-specific antibody IDEC-131 in a nonhuman primate model of skin allotransplantation. J Immunol. 170:2776–82. DOI: 10.4049/jimmunol.170.5.2776. PMID: 12594309.
Article21. Bakela K, Kountourakis N, Aivaliotis M, Athanassakis I. 2015; Soluble MHC-II proteins promote suppressive activity in CD4+ T cells. Immunology. 144:158–69. DOI: 10.1111/imm.12360. PMID: 25053509. PMCID: PMC4264919.22. Won DI, Ham JY, Kim CD, Suh JS, Kim BC. 2015; Benefits of fresh-frozen plasma as a replacement fluid to neutralize ABO antibodies. J Clin Apher. 30:288–96. DOI: 10.1002/jca.21378. PMID: 25546477.
Article23. Scornik JC, Bromberg JS, Norman DJ, Bhanderi M, Gitlin M, Petersen J. 2013; An update on the impact of pre-transplant transfusions and allosensitization on time to renal transplant and on allograft survival. BMC Nephrol. 14:217. DOI: 10.1186/1471-2369-14-217. PMID: 24107093. PMCID: PMC4125965.
Article24. van Twuyver E, Mooijaart RJ, ten Berge IJ, et al. 1991; Pretransplantation blood transfusion revisited. N Engl J Med. 325:1210–3. DOI: 10.1056/NEJM199110243251704. PMID: 1922208.
Article25. Bushell A, Karim M, Kingsley CI, Wood KJ. 2003; Pretransplant blood transfusion without additional immunotherapy generates CD25+ CD4+ regulatory T cells: a potential explanation for the blood-transfusion effect. Transplantation. 76:449–55. DOI: 10.1097/01.TP.0000083043.84630.99. PMID: 12923427.26. Jordan SC, Vo A, Bunnapradist S, et al. 2003; Intravenous immune globulin treatment inhibits crossmatch positivity and allows for successful transplantation of incompatible organs in living-donor and cadaver recipients. Transplantation. 76:631–6. DOI: 10.1097/01.TP.0000080685.31697.FC. PMID: 12973100.27. Santoso S, Kiefel V, Volz H, Mueller-Eckhardt C. 1992; Quantitation of soluble hla class-I antigen in human albumin and immunoglobulin preparations for intravenous use by solid-phase immunoassay. Vox Sang. 62:29–33. DOI: 10.1111/j.1423-0410.1992.tb01163.x. PMID: 1580064.28. Grosse-Wilde H, Blasczyk R, Westhoff U. 1992; Soluble HLA class I and class II concentrations in commercial immunoglobulin preparations. Tissue Antigens. 39:74–7. DOI: 10.1111/j.1399-0039.1992.tb01910.x. PMID: 1574801.29. Gramatges MM, Fani P, Nadeau K, Pereira S, Jeng MR. 2009; Neonatal alloimmune thrombocytopenia and neutropenia associated with maternal human leukocyte antigen antibodies. Pediatr Blood Cancer. 53:97–9. DOI: 10.1002/pbc.21979. PMID: 19229975.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of preexisting human leukocyte antigen donor-specific antibodies especially human leukocyte antigen-DQ on kidney transplant outcome
- Prognostic potential: evaluating graft donor specific antibody and HLA antigen complexes in living-donor liver transplantation
- Causes of Positive Pretransplant Crossmatches in the Absence of Donor-Specific Anti-Human Leukocyte Antigen Antibodies: A Single-Center Experience
- Investigation of non-human leukocyte antigen antibodies and epitope mismatch in kidney transplant recipients with chronic antibody-mediated rejection
- Pretransplant immunologic risk assessment in high baseline ABO antibody titers and donor-specific anti-human leukocyte antigen antibodies in ABO incompatible kidney transplantation