Ann Surg Treat Res.
2020 Jul;99(1):26-36. 10.4174/astr.2020.99.1.26.
Serum level of visfatin can reflect the severity of inflammation in patients with acute cholecystitis
- Affiliations
-
- 1Department of Surgery, Daejeon St. Mary’s Hospital, The Catholic University of Korea, Seoul, Korea
- 2Department of Surgery, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 3Catholic Central Laboratory of Surgery, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 4Department of Surgery, Uijeongbu St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2503457
- DOI: http://doi.org/10.4174/astr.2020.99.1.26
Abstract
- Purpose
Visfatin is a key cytokine released from the pe ripheral blood mononuclear cells (PBMCs) as well as adipose tissue, and it is involved in immune response as well as inflammation. In this study, we investigated whether the serum visfatin level could be a prognostic factor for predicting the severity of inflammation in patients with acute cholecystitis.
Methods
We examined the blood samples and gallbladder specimens from patients who underwent laparoscopic cholecystectomy for either acute (n = 18) or chronic cholecystitis (n = 18). We determined the visfatin levels of these samples using various procedures such as real-time polymerase chain reaction, enzyme-linked immunosorbent assay, western blotting, and immunohistochemistry.
Results
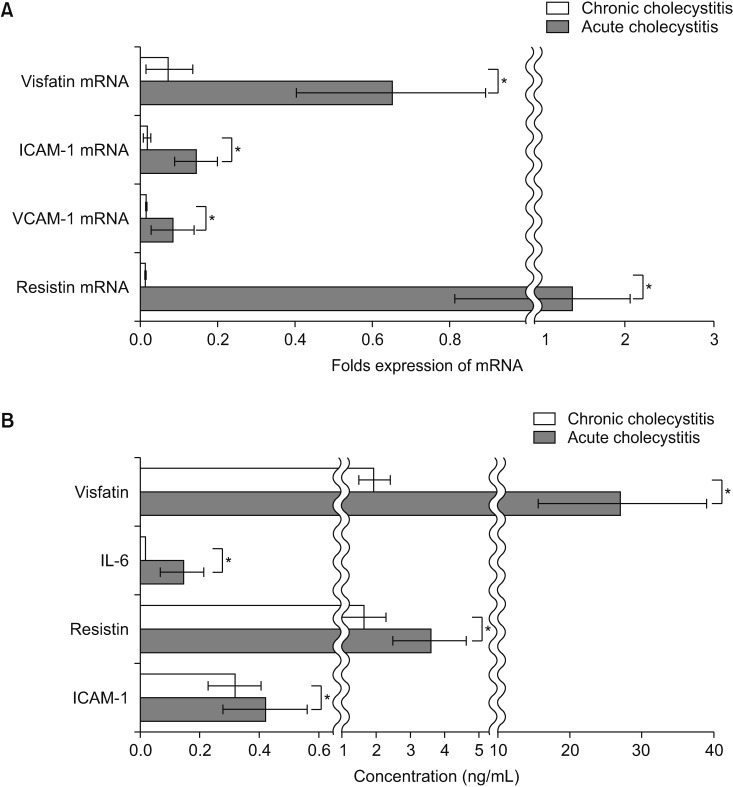
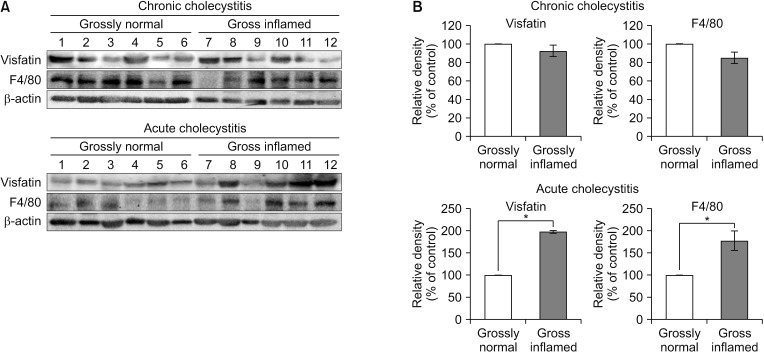
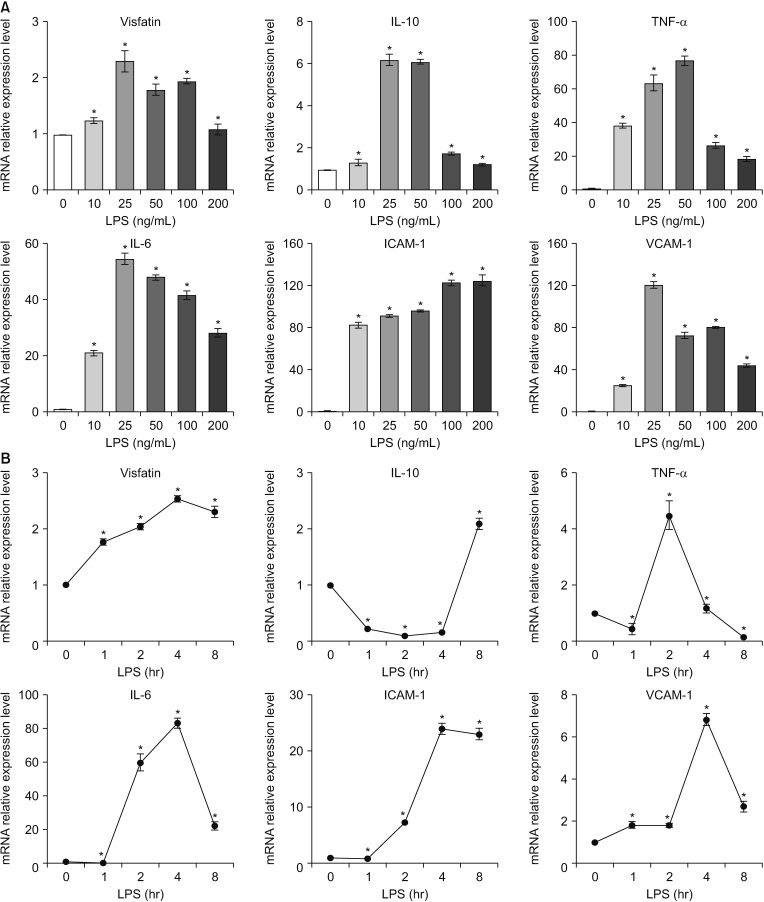
The patients with acute cholecystitis exhibited higher mRNA expression of visfatin in PBMCs, higher serum levels of visfatin, and increased protein expression of visfatin in the gallbladder specimens than in patients with chronic cholecystitis. In the in vitro model of acute cholecystitis, the mRNA expression of visfatin showed the fastest increase among the other pro-inflammatory mediators studied, including interleukin (IL)-10, tumor necrosis factor-, IL-6, intracellular adhesion molecule-1, and ascular cell adhesion molecule-1. Inhibition of visfatin using siRNA abrogated the inhibitory effects of lipopolysaccharide (LPS) on the expression of ABCG1 in GBECs, suggesting that visfatin is significantly involved in the LPS-driven suppression of ABCG1.
Conclusion
Taken together, we concluded that visfatin is a pro-inflammatory mediators that is upregulated during acute cholecystitis and is expected to be increased within a short time after inflammation. Therefore, measuring the serum level of visfatin would be helpful in predicting the inflammatory severity in the patients with acute cholecystitis.
Keyword
Figure
Reference
-
1. Trowbridge RL, Rutkowski NK, Shojania KG. Does this pat ient have acute cholecystitis? JAMA. 2003; 289:80–86. PMID: 12503981.2. Friedman GD. Natural histor y of asymptomat ic and symptomat ic gallstones. Am J Surg. 1993; 165:399–404. PMID: 8480871.3. Sartelli M, Abu-Zidan FM, Catena F, Griffiths EA, Di Saverio S, Coimbra R, et al. Global validation of the WSES Sepsis Severity Score for patients with complicated intra-abdominal infections: a prospective multicentre study (WISS Study). World J Emerg Surg. 2015; 10:61. PMID: 26677396.4. Xie KG, Teng XP, Zhu SY, Qiu XB, Ye XM, Hong XM. Elevated plasma visfatin levels correlate with conversion of laparoscopic cholecystectomy to open surgery in acute cholecystitis. Peptides. 2014; 60:8–12. PMID: 25086268.5. Fukuhara A, Matsuda M, Nishizawa M, Segawa K, Tanaka M, Kishimoto K, et al. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science. 2005; 307:426–430. PMID: 15604363.6. Berndt J, Kloting N, Kralisch S, Kovacs P, Fasshauer M, Schon MR, et al. Plasma visfatin concentrations and fat depot-specific mRNA expression in humans. Diabetes. 2005; 54:2911–2916. PMID: 16186392.7. de Fougerolles AR, Chi-Rosso G, Bajardi A, Gotwals P, Green CD, Koteliansky VE. Global expression analysis of extracellular matrix-integrin interactions in monocytes. Immunity. 2000; 13:749–758. PMID: 11163191.8. Liu K, Li Y, Prabhu V, Young L, Becker KG, Munson PJ, et al. Augmentation in expression of activation-induced genes differentiates memory from naive CD4+ T cells and is a molecular mechanism for enhanced cellular response of memory CD4+ T cells. J Immunol. 2001; 166:7335–7344. PMID: 11390484.9. Luk T, Malam Z, Marshall JC. Pre-B cell colony-enhancing factor (PBEF)/visfatin: a novel mediator of innate immunity. J Leukoc Biol. 2008; 83:804–816. PMID: 18252866.10. Moschen AR, Kaser A, Enrich B, Mosheimer B, Theurl M, Niederegger H, et al. Visfatin, an adipocytokine with proinflammatory and immunomodulating properties. J Immunol. 2007; 178:1748–1758. PMID: 17237424.11. Stofkova A. Resistin and visfatin: regulators of insulin sensitivity, inflammation and immunity. Endocr Regul. 2010; 44:25–36. PMID: 20151765.12. Jia SH, Li Y, Parodo J, Kapus A, Fan L, Rotstein OD, et al. Pre-B cell colony-enhancing factor inhibits neutrophil apoptosis in experimental inflammation and clinical sepsis. J Clin Invest. 2004; 113:1318–1327. PMID: 15124023.13. Kralisch S, Klein J, Lossner U, Bluher M, Paschke R, Stumvoll M, et al. Hormonal regulation of the novel adipocytokine visfatin in 3T3-L1 adipocytes. J Endocrinol. 2005; 185:R1–R8. PMID: 15930160.14. Koczan D, Guthke R, Thiesen HJ, Ibrahim SM, Kundt G, Krentz H, et al. Gene expression profiling of peripheral blood mononuclear leukocytes from psoriasis patients identifies new immune regulatory molecules. Eur J Dermatol. 2005; 15:251–257. PMID: 16048752.15. Nowell MA, Richards PJ, Fielding CA, Ognjanovic S, Topley N, Williams AS, et al. Regulation of pre-B cell colony-enhancing factor by STAT-3-dependent interleukin-6 trans-signaling: implications in the pathogenesis of rheumatoid arthritis. Arthritis Rheum. 2006; 54:2084–2095. PMID: 16802343.16. Ye SQ, Simon BA, Maloney JP, Zambelli-Weiner A, Gao L, Grant A, et al. Pre-B-cell colony-enhancing factor as a potential novel biomarker in acute lung injury. Am J Respir Crit Care Med. 2005; 171:361–370. PMID: 15579727.17. Xiao K, Zou WH, Yang Z, Rehman ZU, Ansari AR, Yuan HR, et al. The role of visfatin on the regulation of inflammation and apoptosis in the spleen of LPS-treated rats. Cell Tissue Res. 2015; 359:605–618. PMID: 25358398.18. Kobayashi K, Kan M, Yamane I, Ishii M, Toyota T. Primary culture of human gallbladder epithelial cells. Gastroenterol Jpn. 1991; 26:363–369. PMID: 1832405.19. Romagnolo DF, Davis CD, Milner JA. Phytoalexins in cancer prevention. Front Biosci (Landmark Ed). 2012; 17:2035–2058. PMID: 22652763.20. Traves PG, Luque A, Hortelano S. Macrophages, inflammation, and tumor suppressors: ARF, a new player in the game. Mediators Inflamm. 2012; 2012:568783. PMID: 23316105.21. Wang SN, Yeh YT, Wang ST, Chuang SC, Wang CL, Yu ML, et al. Visfatin--a proinflammatory adipokine-in gallstone disease. Am J Surg. 2010; 199:459–465. PMID: 19427623.22. Arner P. Visfatin--a true or false trail to type 2 diabetes mellitus. J Clin Endocrinol Metab. 2006; 91:28–30. PMID: 16401830.23. Tilg H, Moschen AR. Role of adiponectin and PBEF/visfatin as regulators of inflammation: involvement in obesity-associated diseases. Clin Sci (Lond). 2008; 114:275–288. PMID: 18194136.24. Graham TE, Yang Q, Bluher M, Hammarstedt A, Ciaraldi TP, Henry RR, et al. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med. 2006; 354:2552–2563. PMID: 16775236.25. Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005; 115:1111–1119. PMID: 15864338.26. Bonfrate L, Wang DQ, Garruti G, Portincasa P. Obesity and the risk and prognosis of gallstone disease and pancreatitis. Best Pract Res Clin Gastroenterol. 2014; 28:623–635. PMID: 25194180.27. Shiffman ML, Sugerman HJ, Kellum JH, Brewer WH, Moore EW. Gallstones in patients with morbid obesity. Relationship to body weight, weight loss and gallbladder bile cholesterol solubility. Int J Obes Relat Metab Disord. 1993; 17:153–158. PMID: 8385075.28. Chen MP, Chung FM, Chang DM, Tsai JC, Huang HF, Shin SJ, et al. Elevated plasma level of visfatin/pre-B cell colony-enhancing factor in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2006; 91:295–299. PMID: 16234302.29. Cottam DR, Mattar SG, Barinas-Mitchell E, Eid G, Kuller L, Kelley DE, et al. The chronic inflammatory hypothesis for the morbidity associated with morbid obesity: implications and effects of weight loss. Obes Surg. 2004; 14:589–600. PMID: 15186624.30. Park Y, Pham TX, Lee J. Lipopolysaccharide represses the expression of ATP-binding cassette transporter G1 and scavenger receptor class B, type I in murine macrophages. Inflamm Res. 2012; 61:465–472. PMID: 22240665.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Elevated Serum Levels of Visfatin in Patients with Henoch-Schonlein Purpura
- A Different View on the Etiopathogenesis of Attention-deficit Hyperactivity Disorder from an Inflammation Perspective
- The Relationship between Plasma Visfatin Level, Obesity and Metabolic Syndrome in Women Without Diabetes
- Correlation of the Serum Nitrate/Nitrite Level with the Severity of Biliary Tract Inflammation
- Acute cholecystitis developed immediately after thoracic kyphoplasty: A case report