J Korean Med Sci.
2020 Jun;35(23):e177. 10.3346/jkms.2020.35.e177.
Intravenous Glucocorticoid Treatment for Korean Graves' Ophthalmopathy Patients
- Affiliations
-
- 1Department of Internal Medicine, Chung-Ang University College of Medicine, Seoul, Korea
- 2Department of Ophthalmology, Chung-Ang University College of Medicine, Seoul, Korea
- KMID: 2502920
- DOI: http://doi.org/10.3346/jkms.2020.35.e177
Abstract
- Background
High-dose intravenous steroids are the first-line treatment for patients with moderate-to-severe and active Graves' ophthalmopathy (GO). We aimed to investigate the response rate of methylprednisolone (MPD) treatment among Korean patients with active moderate-to-severe GO and to identify predictive factors of treatment response.
Methods
This is a retrospective observational study. We included 54 active moderate-to-severe GO patients treated with 4.5 g intravenous MPD over 12 weeks between November 2011 and November 2018. Response was defined as an improvement in at least two of five indicators (clinical activity score [CAS], soft-tissue involvement, exophthalmos, diplopia, and visual acuity) at immediate and 3 months after treatment completion. We examined predictive factors for response using logistic regression analysis.
Results
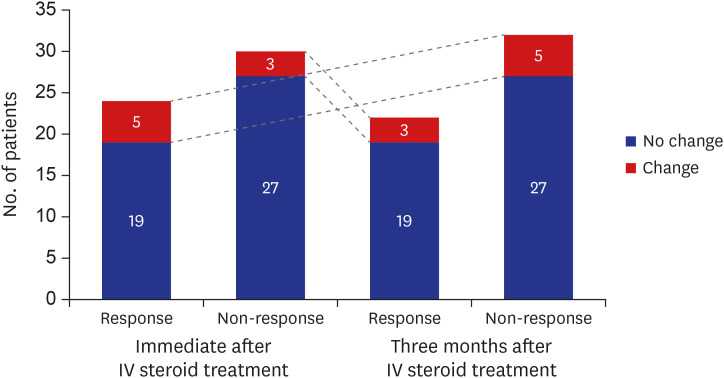
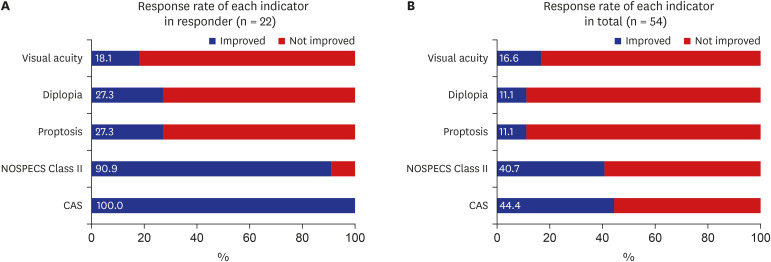
Twenty-four (44.4%) and 22 (40.7%) patients showed response at immediate and 3 months after intravenous (IV) steroid treatment. Of the five ophthalmic parameters, all patients in the responsive group (100.0%) showed a decrease in CAS and 90.9% showed less soft tissue involvement after IV steroid treatment. Among variables, the sum of extraocular muscle width was positively (odds ratio [OR], 1.163; 95% confidence interval [CI], 0.973–1.389; P = 0.096) associated with treatment response. While, the OR of age was 0.918 (95% CI, 0.856–0.985; P = 0.017) and thyrotropin binding inhibitory immunoglobulin (TBII) was 0.921 (95% CI, 0.864–0.982; P = 0.012).
Conclusion
In Korean active moderate-to-severe GO patients, intravenous steroid treatment is not as effective as previously reported. Parameters associated with CAS and soft-tissue involvement were found to be influenced by IV MPD treatment. Extraocular muscle enlargement, younger age and lower TBII are predictive factors for a good steroid treatment response.
Figure
Reference
-
2. Wiersinga WM, Bartalena L. Epidemiology and prevention of Graves' ophthalmopathy. Thyroid. 2002; 12(10):855–860. PMID: 12487767.
Article3. Bartalena L, Baldeschi L, Boboridis K, Eckstein A, Kahaly GJ, Marcocci C, et al. The 2016 European Thyroid Association/European Group on Graves' orbitopathy guidelines for the management of Graves' orbitopathy. Eur Thyroid J. 2016; 5(1):9–26. PMID: 27099835.
Article4. Mourits MP, Prummel MF, Wiersinga WM, Koornneef L. Clinical activity score as a guide in the management of patients with Graves' ophthalmopathy. Clin Endocrinol (Oxf). 1997; 47(1):9–14. PMID: 9302365.
Article5. Zang S, Ponto KA, Kahaly GJ. Clinical review: Intravenous glucocorticoids for Graves' orbitopathy: efficacy and morbidity. J Clin Endocrinol Metab. 2011; 96(2):320–332. PMID: 21239515.6. van Geest RJ, Sasim IV, Koppeschaar HP, Kalmann R, Stravers SN, Bijlsma WR, et al. Methylprednisolone pulse therapy for patients with moderately severe Graves' orbitopathy: a prospective, randomized, placebo-controlled study. Eur J Endocrinol. 2008; 158(2):229–237. PMID: 18230831.
Article7. Macchia PE, Bagattini M, Lupoli G, Vitale M, Vitale G, Fenzi G. High-dose intravenous corticosteroid therapy for Graves' ophthalmopathy. J Endocrinol Invest. 2001; 24(3):152–158. PMID: 11314743.
Article8. Menconi F, Marinò M, Pinchera A, Rocchi R, Mazzi B, Nardi M, et al. Effects of total thyroid ablation versus near-total thyroidectomy alone on mild to moderate Graves' orbitopathy treated with intravenous glucocorticoids. J Clin Endocrinol Metab. 2007; 92(5):1653–1658. PMID: 17299076.
Article9. Bartalena L, Baldeschi L, Dickinson A, Eckstein A, Kendall-Taylor P, Marcocci C, et al. Consensus statement of the European Group on Graves' Orbitopathy (EUGOGO) on management of GO. Eur J Endocrinol. 2008; 158(3):273–285. PMID: 18299459.
Article10. Chun YS, Park HH, Park IK, Moon NJ, Park SJ, Lee JK. Topographic analysis of eyelid position using digital image processing software. Acta Ophthalmol. 2017; 95(7):e625–32. PMID: 28391655.
Article11. Bahn RS, Gorman CA. Choice of therapy and criteria for assessing treatment outcome in thyroid-associated ophthalmopathy. Endocrinol Metab Clin North Am. 1987; 16(2):391–407. PMID: 3319588.
Article12. Eckstein AK, Plicht M, Lax H, Neuhäuser M, Mann K, Lederbogen S, et al. Thyrotropin receptor autoantibodies are independent risk factors for Graves' ophthalmopathy and help to predict severity and outcome of the disease. J Clin Endocrinol Metab. 2006; 91(9):3464–3470. PMID: 16835285.
Article13. Xing L, Ye L, Zhu W, Shen L, Huang F, Jiao Q, et al. Smoking was associated with poor response to intravenous steroids therapy in Graves' ophthalmopathy. Br J Ophthalmol. 2015; 99(12):1686–1691. PMID: 26061160.
Article14. Wang Y, Zhang S, Zhang Y, Liu X, Gu H, Zhong S, et al. A single-center retrospective study of factors related to the effects of intravenous glucocorticoid therapy in moderate-to-severe and active thyroid-associated ophthalmopathy. BMC Endocr Disord. 2018; 18(1):13. PMID: 29463244.
Article15. Hu S, Wang Y, He M, Zhang M, Ding X, Shi B. Factors associated with the efficacy of intravenous methylprednisolone in moderate-to-severe and active thyroid-associated ophthalmopathy: a single-centre retrospective study. Clin Endocrinol (Oxf). 2019; 90(1):175–183. PMID: 30229982.
Article16. Xu L, Li L, Xie C, Guan M, Xue Y. Thickness of extraocular muscle and orbital fat in MRI predicts response to glucocorticoid therapy in Graves' ophthalmopathy. Int J Endocrinol. 2017; 2017:3196059. PMID: 28845157.
Article17. Kahaly GJ, Pitz S, Hommel G, Dittmar M. Randomized, single blind trial of intravenous versus oral steroid monotherapy in Graves' orbitopathy. J Clin Endocrinol Metab. 2005; 90(9):5234–5240. PMID: 15998777.18. Aktaran S, Akarsu E, Erbağci I, Araz M, Okumuş S, Kartal M. Comparison of intravenous methylprednisolone therapy vs. oral methylprednisolone therapy in patients with Graves' ophthalmopathy. Int J Clin Pract. 2007; 61(1):45–51. PMID: 16889639.
Article19. Bartalena L, Veronesi G, Krassas GE, Wiersinga WM, Marcocci C, Marinò M, et al. Does early response to intravenous glucocorticoids predict the final outcome in patients with moderate-to-severe and active Graves' orbitopathy? J Endocrinol Invest. 2017; 40(5):547–553. PMID: 28176220.
Article20. Wakelkamp IM, Bakker O, Baldeschi L, Wiersinga WM, Prummel MF. TSH-R expression and cytokine profile in orbital tissue of active vs. inactive Graves' ophthalmopathy patients. Clin Endocrinol (Oxf). 2003; 58(3):280–287. PMID: 12608932.
Article21. Iyer S, Bahn R. Immunopathogenesis of Graves' ophthalmopathy: the role of the TSH receptor. Best Pract Res Clin Endocrinol Metab. 2012; 26(3):281–289. PMID: 22632365.
Article22. Gerding MN, van der Meer JW, Broenink M, Bakker O, Wiersinga WM, Prummel MF. Association of thyrotrophin receptor antibodies with the clinical features of Graves' ophthalmopathy. Clin Endocrinol (Oxf). 2000; 52(3):267–271. PMID: 10718823.
Article23. Lytton SD, Ponto KA, Kanitz M, Matheis N, Kohn LD, Kahaly GJ. A novel thyroid stimulating immunoglobulin bioassay is a functional indicator of activity and severity of Graves' orbitopathy. J Clin Endocrinol Metab. 2010; 95(5):2123–2131. PMID: 20237164.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Euthyroid Graves' Ophthalmopathy with Negative Autoantibodies
- Predictive Factor for Therapeutic Results after Intravenous Glucocorticoid Therapy in Thyroid-Associated Ophthalmopathy
- Treatment of Graves' Ophthalmopathy
- A Case of Graves' Disease Showing a Triad of Ophthalmopathy, Pretibial Myxedema and Thyroid Acropachy
- Development of Graves' Ophthalmopathy after Radioactive Iodine Ablation Using Recombinant Human Thyrotropin for Incidentally Discovered Papillary Thyroid Carcinoma