J Korean Med Sci.
2020 Jun;35(23):e168. 10.3346/jkms.2020.35.e168.
A Rare Case of Essential Thrombocythemia with Coexisting JAK2 and MPL Driver Mutations
- Affiliations
-
- 1Department of Laboratory Medicine and Genetics, Soonchunhyang University Bucheon Hospital, Soonchunhyang University College of Medicine, Bucheon, Korea
- 2Division of Hematology & Oncology, Department of Internal Medicine, Soonchunhyang University Bucheon Hospital, Soonchunhyang University College of Medicine, Bucheon, Korea
- KMID: 2502919
- DOI: http://doi.org/10.3346/jkms.2020.35.e168
Abstract
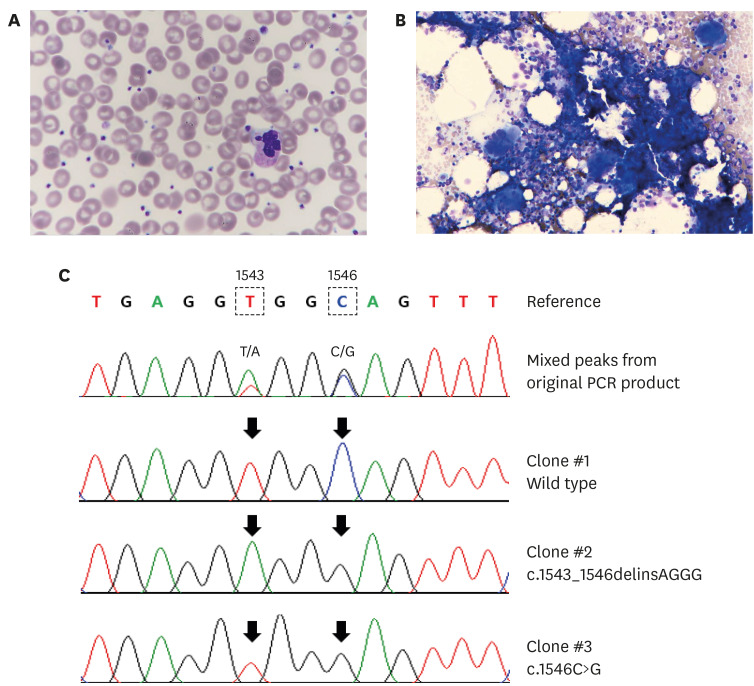
- Philadelphia-negative (Ph−) classical myeloproliferative neoplasms (MPNs) include polycythemia vera, essential thrombocythemia (ET), and primary myelofibrosis. Somatic driver mutations in the JAK2, CALR, and MPL genes serve as major diagnostic criteria of the Ph− MPNs and these mutations occur in a mutually exclusive manner. In this report, we describe the first case of ET harboring double mutations in JAK2 V617F and MPL. For MPL, the patient had multiple clones of MPL mutations: c.1543_1546delinsAGGG (p.Trp515_Gln516delinsArgGlu) and c.1546C>G (p.Gln516Glu). The JAK2 V617F allele burden in our patient is very low (4%) compared to the relatively high (17%–78%) allele frequency of MPL mutations. The low JAK2 mutant burden might be explained by preexisting clonal hematopoiesis before overt signs of MPNs, followed by the acquisition of a second oncogenic mutation of CALR or MPL leading to the MPN phenotype. This highlights that screening for a second driver mutation should be considered in patients with a low JAK2 mutant burden by reporting a 57-year-old Korean man with ET.
Keyword
Figure
Cited by 1 articles
-
JAK2 V617F 양성 급성골수성백혈병의 임상병리학적 특징 2예
Youngeun Lee, Ji Yun Lee, Jeong-Ok Lee, Soo-Mee Bang, Sang Mee Hwang
Lab Med Online. 2022;12(1):53-57. doi: 10.47429/lmo.2022.12.1.53.
Reference
-
1. Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC;2017.2. Lim Y, Lee JO, Bang SM. Incidence, survival and prevalence statistics of classical myeloproliferative neoplasm in Korea. J Korean Med Sci. 2016; 31(10):1579–1585. PMID: 27550486.
Article3. Vainchenker W, Kralovics R. Genetic basis and molecular pathophysiology of classical myeloproliferative neoplasms. Blood. 2017; 129(6):667–679. PMID: 28028029.
Article4. Mansier O, Luque Paz D, Ianotto JC, Le Bris Y, Chauveau A, Boyer F, et al. Clinical and biological characterization of MPN patients harboring two driver mutations, a French intergroup of myeloproliferative neoplasms (FIM) study. Am J Hematol. 2018; 93(4):E84–E86. PMID: 29266414.
Article5. De Roeck L, Michaux L, Debackere K, Lierman E, Vandenberghe P, Devos T. Coexisting driver mutations in MPN: clinical and molecular characteristics of a series of 11 patients. Hematology. 2018; 23(10):785–792. PMID: 29993347.
Article6. Kang MG, Choi HW, Lee JH, Choi YJ, Choi HJ, Shin JH, et al. Coexistence of JAK2 and CALR mutations and their clinical implications in patients with essential thrombocythemia. Oncotarget. 2016; 7(35):57036–57049. PMID: 27486987.
Article7. Park SH, Chi HS, Cho YU, Jang S, Park CJ, Kim DY, et al. Two cases of myeloproliferative neoplasm with a concurrent JAK2 (V617F) mutation and BCR/ABL translocation without chronic myelogenous leukemia phenotype acquisition during hydroxyurea treatment. Ann Lab Med. 2013; 33(3):229–232. PMID: 23667855.8. Jeromin S, Kohlmann A, Meggendorfer M, Schindela S, Perglerová K, Nadarajah N, et al. Next-generation deep-sequencing detects multiple clones of CALR mutations in patients with BCR-ABL1 negative MPN. Leukemia. 2016; 30(4):973–976. PMID: 26220041.
Article9. Kim BH, Cho YU, Bae MH, Jang S, Seo EJ, Chi HS, et al. JAK2 V617F, MPL, and CALR mutations in Korean patients with essential thrombocythemia and primary myelofibrosis. J Korean Med Sci. 2015; 30(7):882–888. PMID: 26130950.
Article10. Kim SY, Im K, Park SN, Kwon J, Kim JA, Lee DS. CALR, JAK2, and MPL mutation profiles in patients with four different subtypes of myeloproliferative neoplasms: primary myelofibrosis, essential thrombocythemia, polycythemia vera, and myeloproliferative neoplasm, unclassifiable. Am J Clin Pathol. 2015; 143(5):635–644. PMID: 25873496.11. Nielsen C, Bojesen SE, Nordestgaard BG, Kofoed KF, Birgens HS. JAK2V617F somatic mutation in the general population: myeloproliferative neoplasm development and progression rate. Haematologica. 2014; 99(9):1448–1455. PMID: 24907356.
Article12. Jang MA, Choi CW. Recent insights regarding the molecular basis of myeloproliferative neoplasms. Korean J Intern Med. 2020; 35(1):1–11. PMID: 31778606.
Article13. Xie J, Chen X, Gao F, Hou R, Tian T, Zhang Y, et al. Two activating mutations of MPL in triple-negative myeloproliferative neoplasms. Cancer Med. 2019; 8(11):5254–5263. PMID: 31294534.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- JAK2 V617F and MPL W515L/K Mutations in Korean Patients with Essential Thrombocythemia
- Frequency and Clinicohematologic Characteristics of MPL W515 Mutations in Patients with Myeloproliferative Neoplasms
- Acute Ischemic Stroke Associated With Essential Thrombocythemia and JAK2 Mutation
- JAK2 V617F, MPL, and CALR Mutations in Korean Patients with Essential Thrombocythemia and Primary Myelofibrosis
- Advances in the Diagnosis of Myeloproliferative Neoplasms