Korean J Physiol Pharmacol.
2020 May;24(3):241-248. 10.4196/kjpp.2020.24.3.241.
Heat shock protein 90 inhibitor AUY922 attenuates platelet-derived growth factor-BB-induced migration and proliferation of vascular smooth muscle cells
- Affiliations
-
- 1Department of Sports Medicine and Science in Graduate School, Konkuk University
- 2Research & Development Center, UMUST R&D Corporation, Seoul 05029, Korea
- 3Department of Nuclear Medicine, Ewha Womans University Seoul Hospital, Ewha Womans University College of Medicine, Seoul 07804, Korea
- 4Department of Nuclear Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul 05505, Korea
- KMID: 2502506
- DOI: http://doi.org/10.4196/kjpp.2020.24.3.241
Abstract
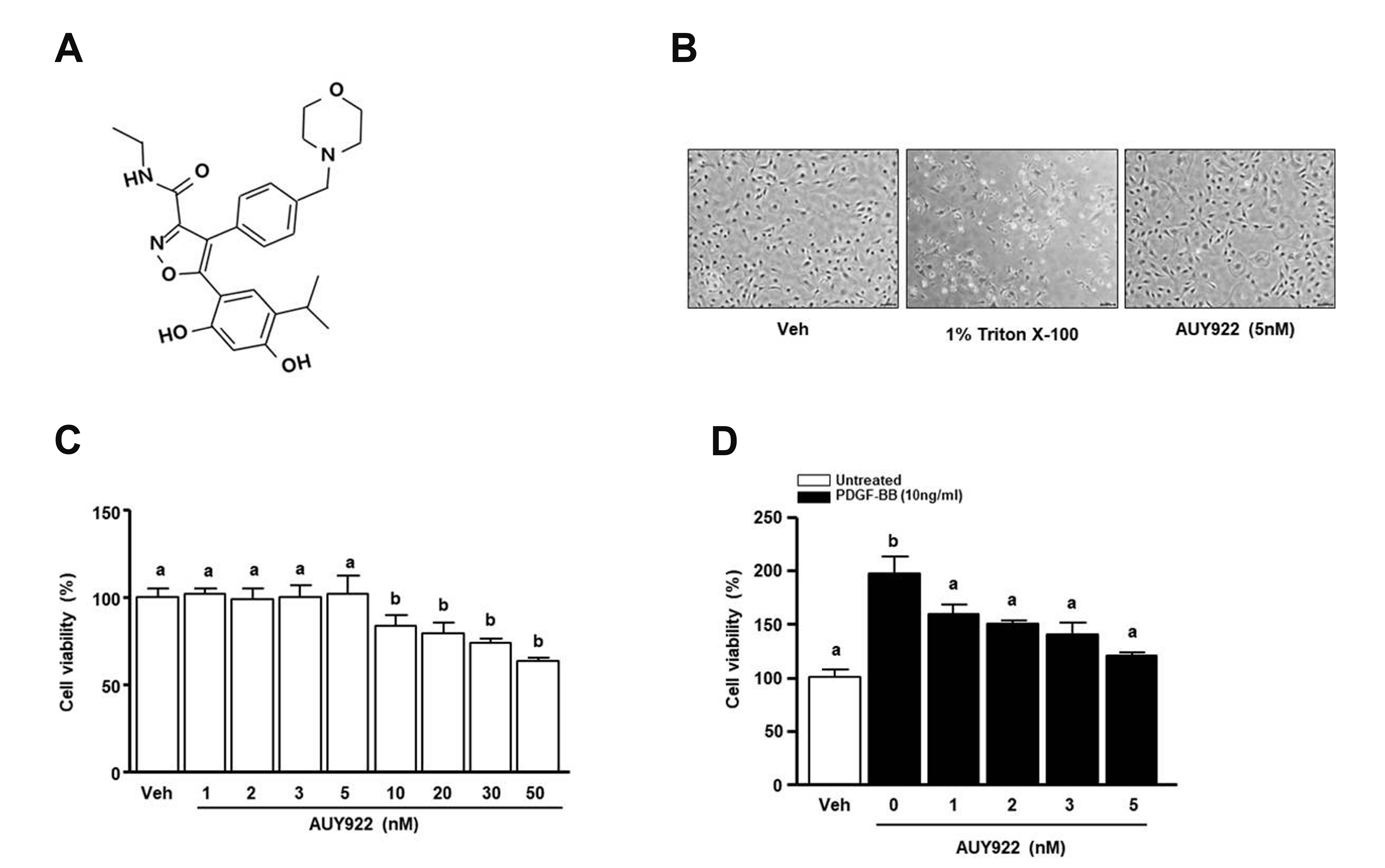
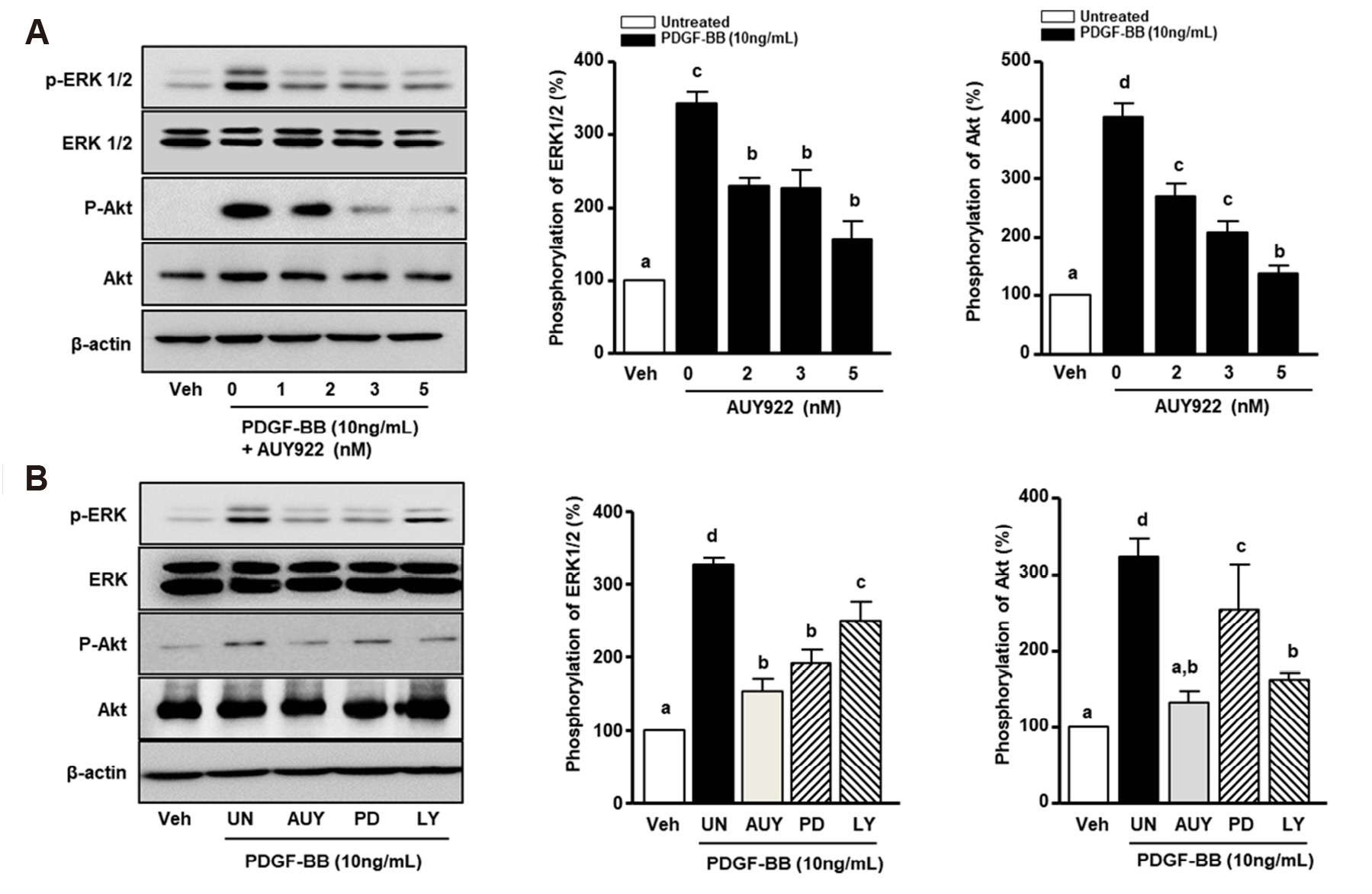
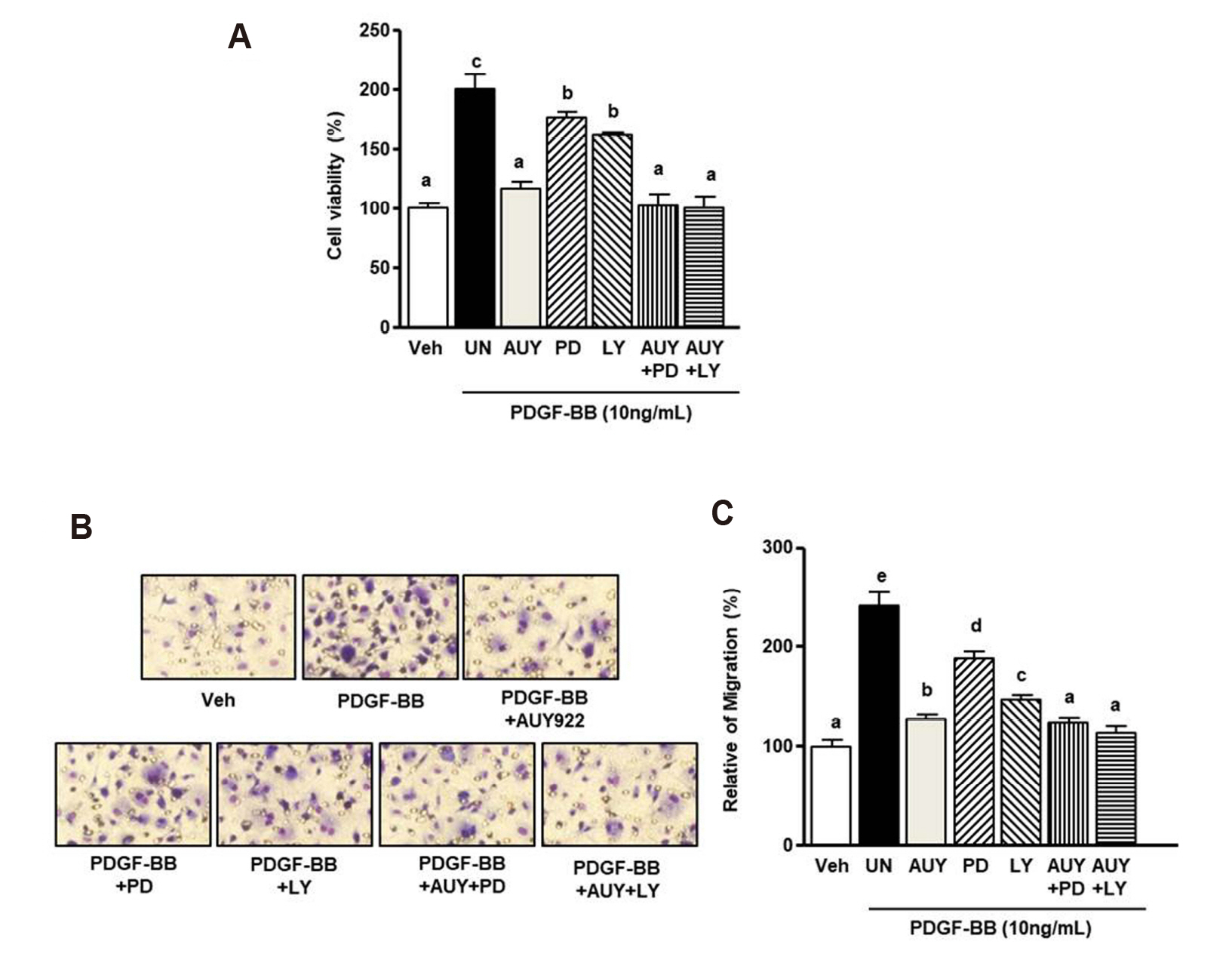
- Luminespib (AUY922), a heat shock proteins 90 inhibitor, has anti-neoplastic and antitumor effects. However, it is not clear whether AUY922 affects events in vascular diseases. We investigated the effects of AUY922 on the platelet-derived growth factor (PDGF)-BB-stimulated proliferation and migration of vascular smooth muscle cells (VSMC). VSMC viability was detected using the XTT (2,3-bis-(2-methoxy- 4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide) reagent. To detect the attenuating effects of AUY922 on PDGF-BB-induced VSMCs migration in vitro, we performed the Boyden chamber and scratch wound healing assays. To identify AUY922- mediated changes in the signaling pathway, the phosphorylation of protein kinase B (Akt) and extracellular signal-regulated kinase (ERK) 1/2 was analyzed by immunoblotting. The inhibitory effects of AUY922 on migration and proliferation ex vivo were tested using an aortic ring assay. AUY922 was not cytotoxic at concentrations up to 5 nM. PDGF-BB-induced VSMC proliferation, migration, and sprout outgrowth were significantly decreased by AUY922 in a dose-dependent manner. AUY922 significantly reduced the PDGF-BB-stimulated phosphorylation of Akt and ERK1/2. Furthermore, PD98059 (a selective ERK1/2 inhibitor) and LY294002 (a selective Akt inhibitor) decreased VSMC migration and proliferation by inhibiting phosphorylation of Akt and ERK1/2. Greater attenuation of PDGF-BB-induced cell viability and migration was observed upon treatment with PD98059 or LY294002 in combination with AUY922. AUY922 showed anti-proliferation and anti-migration effects towards PDGF-BBinduced VSMCs by regulating the phosphorylation of ERK1/2 and Akt. Thus, AUY922 is a candidate for the treatment of atherosclerosis and restenosis.
Keyword
Figure
Reference
-
1. Frostegård J. 2013; Immunity, atherosclerosis and cardiovascular disease. BMC Med. 11:117. DOI: 10.1186/1741-7015-11-117. PMID: 23635324. PMCID: PMC3658954.
Article2. Tousoulis D, Charakida M, Stefanadis C. 2008; Endothelial function and inflammation in coronary artery disease. Postgrad Med J. 84:368–371. DOI: 10.1136/hrt.2005.066936. PMCID: PMC1860901.
Article3. Chaabane C, Otsuka F, Virmani R, Bochaton-Piallat ML. 2013; Biological responses in stented arteries. Cardiovasc Res. 99:353–363. DOI: 10.1093/cvr/cvt115. PMID: 23667187.
Article4. Won KJ, Jung SH, Lee CK, Na HR, Lee KP, Lee DY, Park ES, Choi WS, Shim SB, Kim B. 2013; DJ-1/park7 protects against neointimal formation via the inhibition of vascular smooth muscle cell growth. Cardiovasc Res. 97:553–561. DOI: 10.1093/cvr/cvs363. PMID: 23230227.
Article5. Casscells W. 1991; Smooth muscle cell growth factors. Prog Growth Factor Res. 3:177–206. DOI: 10.1016/0955-2235(91)90006-P. PMID: 1811790.
Article6. Jawien A, Bowen-Pope DF, Lindner V, Schwartz SM, Clowes AW. 1992; Platelet-derived growth factor promotes smooth muscle migration and intimal thickening in a rat model of balloon angioplasty. J Clin Invest. 89:507–511. DOI: 10.1172/JCI115613. PMID: 1531345. PMCID: PMC442880.
Article7. Campbell M, Trimble ER. 2005; Modification of PI3K- and MAPK-dependent chemotaxis in aortic vascular smooth muscle cells by protein kinase CbetaII. Circ Res. 96:197–206. DOI: 10.1161/01.RES.0000152966.88353.9d. PMID: 15591231.8. Moser C, Lang SA, Stoeltzing O. 2009; Heat-shock protein 90 (Hsp90) as a molecular target for therapy of gastrointestinal cancer. Anticancer Res. 29:2031–2042. PMID: 19528462.9. Andrae J, Gallini R, Betsholtz C. 2008; Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 22:1276–1312. DOI: 10.1101/gad.1653708. PMID: 18483217. PMCID: PMC2732412.
Article10. Zhao Z, Wang Y, Li S, Liu S, Liu Y, Yu Y, Yang F, Xu Z, Wang G. 2019; HSP90 inhibitor 17-DMAG effectively alleviated the progress of thoracic aortic dissection by suppressing smooth muscle cell phenotypic switch. Am J Transl Res. 11:509–518. PMID: 30788006. PMCID: PMC6357309.11. Kim J, Jang SW, Park E, Oh M, Park S, Ko J. 2014; The role of heat shock protein 90 in migration and proliferation of vascular smooth muscle cells in the development of atherosclerosis. J Mol Cell Cardiol. 72:157–167. DOI: 10.1016/j.yjmcc.2014.03.008. PMID: 24650873.
Article12. Okamoto J, Mikami I, Tominaga Y, Kuchenbecker KM, Lin YC, Bravo DT, Clement G, Yagui-Beltran A, Ray MR, Koizumi K, He B, Jablons DM. 2008; Inhibition of Hsp90 leads to cell cycle arrest and apoptosis in human malignant pleural mesothelioma. J Thorac Oncol. 3:1089–1095. DOI: 10.1097/JTO.0b013e3181839693. PMID: 18827603. PMCID: PMC2656438.
Article13. Chen Y, Wang X, Cao C, Wang X, Liang S, Peng C, Fu L, He G. 2017; Inhibition of HSP90 sensitizes a novel Raf/ERK dual inhibitor CY-9d in triple-negative breast cancer cells. Oncotarget. 8:104193–104205. DOI: 10.18632/oncotarget.22119. PMID: 29262632. PMCID: PMC5732798.
Article14. Baek S, Lee KP, Cui L, Ryu Y, Hong JM, Kim J, Jung SH, Bae YM, Won KJ, Kim B. 2017; Low-power laser irradiation inhibits PDGF-BB-induced migration and proliferation via apoptotic cell death in vascular smooth muscle cells. Lasers Med Sci. 32:2121–2127. DOI: 10.1007/s10103-017-2338-z. PMID: 28983687.
Article15. Li Y, Zhang T, Schwartz SJ, Sun D. 2009; New developments in Hsp90 inhibitors as anti-cancer therapeutics: mechanisms, clinical perspective and more potential. Drug Resist Updat. 12:17–27. DOI: 10.1016/j.drup.2008.12.002. PMID: 19179103. PMCID: PMC2692088.
Article16. Dzau VJ, Braun-Dullaeus RC, Sedding DG. 2002; Vascular proliferation and atherosclerosis: new perspectives and therapeutic strategies. Nat Med. 8:1249–1256. DOI: 10.1038/nm1102-1249. PMID: 12411952.
Article17. Kang H, Ahn DH, Pak JH, Seo KH, Baek NI, Jang SW. 2016; Magnobovatol inhibits smooth muscle cell migration by suppressing PDGF-Rβ phosphorylation and inhibiting matrix metalloproteinase-2 expression. Int J Mol Med. 37:1239–1246. DOI: 10.3892/ijmm.2016.2548. PMID: 27049716. PMCID: PMC4829143.
Article18. Millette E, Rauch BH, Defawe O, Kenagy RD, Daum G, Clowes AW. 2005; Platelet-derived growth factor-BB-induced human smooth muscle cell proliferation depends on basic FGF release and FGFR-1 activation. Circ Res. 96:172–179. DOI: 10.1161/01.RES.0000154595.87608.db. PMID: 15625285.
Article19. Raica M, Cimpean AM. 2010; Platelet-derived growth factor (PDGF)/PDGF receptors (PDGFR) axis as target for antitumor and antiangiogenic therapy. Pharmaceuticals (Basel). 3:572–599. DOI: 10.3390/ph3030572. PMID: 27713269. PMCID: PMC4033970.
Article20. Vabulas RM, Raychaudhuri S, Hayer-Hartl M, Hartl FU. 2010; Protein folding in the cytoplasm and the heat shock response. Cold Spring Harb Perspect Biol. 2:a004390. DOI: 10.1101/cshperspect.a004390. PMID: 21123396. PMCID: PMC2982175.
Article21. Trepel J, Mollapour M, Giaccone G, Neckers L. 2010; Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer. 10:537–549. DOI: 10.1038/nrc2887. PMID: 20651736. PMCID: PMC6778733.
Article22. Jiao Y, Ou W, Meng F, Zhou H, Wang A. 2011; Targeting HSP90 in ovarian cancers with multiple receptor tyrosine kinase coactivation. Mol Cancer. 10:125. DOI: 10.1186/1476-4598-10-125. PMID: 21962244. PMCID: PMC3196924.
Article23. Porsch H, Mehić M, Olofsson B, Heldin P, Heldin CH. 2014; Platelet-derived growth factor β-receptor, transforming growth factor β type I receptor, and CD44 protein modulate each other's signaling and stability. J Biol Chem. 289:19747–19757. DOI: 10.1074/jbc.M114.547273. PMID: 24860093. PMCID: PMC4094084.
Article24. Won KJ, Park SH, Park T, Lee CK, Lee HM, Choi WS, Kim SJ, Park PJ, Jang HK, Kim SH, Kim B. 2008; Cofilin phosphorylation mediates proliferation in response to platelet-derived growth factor-BB in rat aortic smooth muscle cells. J Pharmacol Sci. 108:372–329. DOI: 10.1254/jphs.FP0072354. PMID: 19023180.
Article25. Namkoong S, Kim CK, Cho YL, Kim JH, Lee H, Ha KS, Choe J, Kim PH, Won MH, Kwon YG, Shim EB, Kim YM. 2009; Forskolin increases angiogenesis through the coordinated cross-talk of PKA-dependent VEGF expression and Epac-mediated PI3K/Akt/eNOS signaling. Cell Signal. 21:906–915. DOI: 10.1016/j.cellsig.2009.01.038. PMID: 19385062.
Article26. ten Freyhaus H, Huntgeburth M, Wingler K, Schnitker J, Bäumer AT, Vantler M, Bekhite MM, Wartenberg M, Sauer H, Rosenkranz S. 2006; Novel Nox inhibitor VAS2870 attenuates PDGF-dependent smooth muscle cell chemotaxis, but not proliferation. Cardiovasc Res. 71:331–341. DOI: 10.1016/j.cardiores.2006.01.022. PMID: 16545786.27. Oliveira JSS, Santos GDS, Moraes JA, Saliba AM, Barja-Fidalgo TC, Mattos-Guaraldi AL, Nagao PE. 2018; Reactive oxygen species generation mediated by NADPH oxidase and PI3K/Akt pathways contribute to invasion of Streptococcus agalactiae in human endothelial cells. Mem Inst Oswaldo Cruz. 113:e140421. DOI: 10.1590/0074-02760170421. PMID: 29641644. PMCID: PMC5887998.
Article28. Nguyen Thi PA, Chen MH, Li N, Zhuo XJ, Xie L. 2016; PD98059 protects brain against cells death resulting from ROS/ERK activation in a cardiac arrest rat model. Oxid Med Cell Longev. 2016:3723762. DOI: 10.1155/2016/3723762. PMID: 27069530. PMCID: PMC4812463.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Olibanum Extract Inhibits Vascular Smooth Muscle Cell Migration and Proliferation in Response to Platelet-Derived Growth Factor
- Artemisinin attenuates platelet-derived growth factor BB-induced migration of vascular smooth muscle cells
- Propofol Inhibits Platelet-derived Growth Factor-stimulated Migration in Rat Aortic Smooth Muscle Cells
- Morphological Analysis of Intimal Hyperplasia in Allografted Aorta of Rat
- The Effects of Cilostazol on Proliferation of Vascular Smooth Muscle Cells and Expression of iNOS and p21