Ann Surg Treat Res.
2020 Jun;98(6):332-339. 10.4174/astr.2020.98.6.332.
Klotho as a potential predictor of deceased donor kidney transplantation outcomes
- Affiliations
-
- 1Department of Surgery, Chung-Ang University College of Medicine, Seoul, Korea
- 2Department of Surgery, Seoul National University College of Medicine, Seoul, Korea
- 3Transplantation Research Institute, Medical Research Center, Seoul National University College of Medicine, Seoul, Korea
- KMID: 2502119
- DOI: http://doi.org/10.4174/astr.2020.98.6.332
Abstract
- Purpose
Klotho is an antiaging factor mainly produced by renal tubular cells. Klotho is reportedly decreased in an animal model of acute kidney injury and patients with chronic kidney disease. However, information on Klotho expression after kidney transplantation is limited. We analyzed the correlation between donor Klotho expression and clinical outcomes of kidney transplantation.
Methods
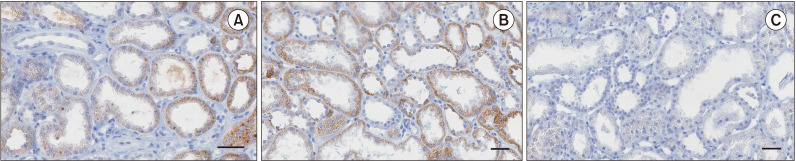
Sixty patients who underwent deceased donor kidney transplantation between March 2015 and October 2017 were enrolled. Serum and tissue Klotho expression levels were measured by enzyme-linked immunosorbent assay and immunohistochemistry, respectively. Graft function was assessed by estimated glomerular filtration rate (eGFR).
Results
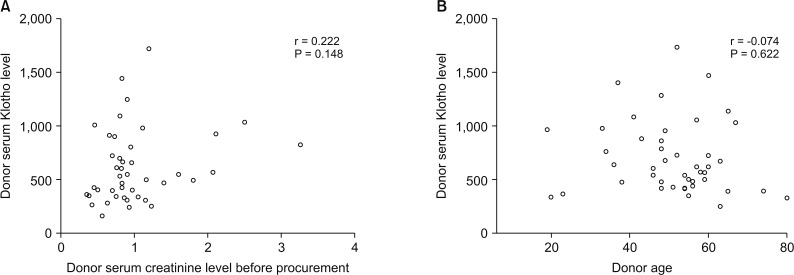
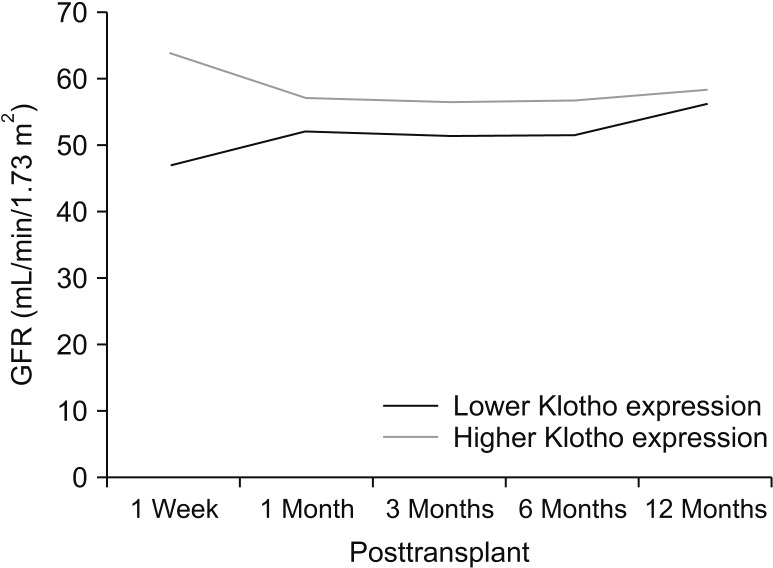
Patients were divided into 2 groups according to donor Klotho expression in renal tissues. A greater improvement in eGFR was observed at 1 week after transplantation in patients receiving kidneys with higher Klotho expression (47.5 ± 21.9 mL/min/1.73 m2 vs. 63.9 ± 28.2 mL/min/1.73 m2, P = 0.030). Patients were also classified into 2 groups according to donor serum Klotho level. There was a tendency for a higher eGFR at 12 months after transplantation in patients receiving kidneys from donors with a higher Klotho level (51.0 ± 18.0 mL/min/1.73 m2 vs. 61.2 ± 16.5 mL/min/1.73 m2, P = 0.059). When subgrouped into patients with or without biopsy-proven acute rejection, 12-month eGFR remained higher in patients receiving kidneys from donors with higher serum Klotho.
Conclusion
Our data demonstrated that donor tissue expression of Klotho correlated with early recovery of eGFR after kidney transplantation. Donor serum Klotho level tended to be associated with posttransplant 12-month eGFR. Donor Klotho expression might be a new predictor for deceased donor kidney transplantation outcome.
Keyword
Figure
Reference
-
1. Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997; 390:45–51. PMID: 9363890.2. Hu MC, Kuro-o M, Moe OW. Renal and extrarenal actions of Klotho. Semin Nephrol. 2013; 33:118–129. PMID: 23465499.3. Olauson H, Mencke R, Hillebrands JL, Larsson TE. Tissue expression and source of circulating αKlotho. Bone. 2017; 100:19–35. PMID: 28323144.4. Deng G, Liu D. Klotho: a promising biomarker closely related to kidney transplant. Exp Clin Transplant. 2018; 16:253–258. PMID: 29676702.5. Xu Y, Sun Z. Molecular basis of Klotho: from gene to function in aging. Endocr Rev. 2015; 36:174–193. PMID: 25695404.6. Hu MC, Shi M, Zhang J, Quinones H, Kuro-o M, Moe OW. Klotho deficiency is an early biomarker of renal ischemia-reperfusion injury and its replacement is protective. Kidney Int. 2010; 78:1240–1251. PMID: 20861825.7. Izquierdo MC, Perez-Gomez MV, Sanchez-Nino MD, Sanz AB, Ruiz-Andres O, Poveda J, et al. Klotho, phosphate and inflammation/ageing in chronic kidney disease. Nephrol Dial Transplant. 2012; 27 Suppl 4:iv6–iv10. PMID: 23258814.8. Moreno JA, Izquierdo MC, Sanchez-Nino MD, Suarez-Alvarez B, Lopez-Larrea C, Jakubowski A, et al. The inflammatory cytokines TWEAK and TNFα reduce renal klotho expression through NFκB. J Am Soc Nephrol. 2011; 22:1315–1325. PMID: 21719790.9. Pavik I, Jaeger P, Ebner L, Wagner CA, Petzold K, Spichtig D, et al. Secreted Klotho and FGF23 in chronic kidney disease Stage 1 to 5: a sequence suggested from a cross-sectional study. Nephrol Dial Transplant. 2013; 28:352–359. PMID: 23129826.10. Barker SL, Pastor J, Carranza D, Quinones H, Griffith C, Goetz R, et al. The demonstration of αKlotho deficiency in human chronic kidney disease with a novel synthetic antibody. Nephrol Dial Transplant. 2015; 30:223–233. PMID: 25324355.11. Kimura T, Akimoto T, Watanabe Y, Kurosawa A, Nanmoku K, Muto S, et al. Impact of renal transplantation and nephrectomy on urinary soluble Klotho protein. Transplant Proc. 2015; 47:1697–1699. PMID: 26293036.12. Leone F, Lofaro D, Gigliotti P, Perri A, Vizza D, Toteda G, et al. Soluble Klotho levels in adult renal transplant recipients are modulated by recombinant human erythropoietin. J Nephrol. 2014; 27:577–585. PMID: 24760622.13. Castellano G, Intini A, Stasi A, Divella C, Gigante M, Pontrelli P, et al. Complement modulation of anti-aging factor Klotho in ischemia/reperfusion injury and delayed graft function. Am J Transplant. 2016; 16:325–333. PMID: 26280899.14. Lim K, Groen A, Molostvov G, Lu T, Lilley KS, Snead D, et al. α-Klotho expression in human tissues. J Clin Endocrinol Metab. 2015; 100:E1308–E1318. PMID: 26280509.15. Cho NJ, Han DJ, Lee JH, Jang SH, Kang JS, Gil HW, et al. Soluble Klotho as a marker of renal fibrosis and podocyte injuries in human kidneys. PLoS One. 2018; 13:e0194617. PMID: 29590173.16. Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008; 8:753–760. PMID: 18294345.17. Denic A, Alexander MP, Kaushik V, Lerman LO, Lieske JC, Stegall MD, et al. Detection and clinical patterns of nephron hypertrophy and nephrosclerosis among apparently healthy adults. Am J Kidney Dis. 2016; 68:58–67. PMID: 26857648.18. Liapis H, Gaut JP, Klein C, Bagnasco S, Kraus E, Farris AB 3rd, et al. Banff histopathological consensus criteria for preimplantation kidney biopsies. Am J Transplant. 2017; 17:140–150. PMID: 27333454.19. Mundt HM, Yard BA, Kramer BK, Benck U, Schnulle P. Optimized donor management and organ preservation before kidney transplantation. Transpl Int. 2016; 29:974–984. PMID: 26563531.20. Ugarte R, Kraus E, Montgomery RA, Burdick JF, Ratner L, Haas M, et al. Excellent outcomes after transplantation of deceased donor kidneys with high terminal creatinine and mild pathologic lesions. Transplantation. 2005; 80:794–800. PMID: 16210967.21. Morgan C, Martin A, Shapiro R, Randhawa PS, Kayler LK. Outcomes after transplantation of deceased-donor kidneys with rising serum creatinine. Am J Transplant. 2007; 7:1288–1292. PMID: 17359500.22. Karpinski J, Lajoie G, Cattran D, Fenton S, Zaltzman J, Cardella C, et al. Outcome of kidney transplantation from high-risk donors is determined by both structure and function. Transplantation. 1999; 67:1162–1167. PMID: 10232568.23. Randhawa PS, Minervini MI, Lombardero M, Duquesnoy R, Fung J, Shapiro R, et al. Biopsy of marginal donor kidneys: correlation of histologic findings with graft dysfunction. Transplantation. 2000; 69:1352–1357. PMID: 10798753.24. Escofet X, Osman H, Griffiths DF, Woydag S, Adam Jurewicz W. The presence of glomerular sclerosis at time zero has a significant impact on function after cadaveric renal transplantation. Transplantation. 2003; 75:344–346. PMID: 12589156.25. Gaber LW, Moore LW, Alloway RR, Amiri MH, Vera SR, Gaber AO. Glomerulosclerosis as a determinant of posttransplant function of older donor renal allografts. Transplantation. 1995; 60:334–339. PMID: 7652761.26. Pokorna E, Vitko S, Chadimova M, Schuck O, Ekberg H. Proportion of glomerulosclerosis in procurement wedge renal biopsy cannot alone discriminate for acceptance of marginal donors. Transplantation. 2000; 69:36–43. PMID: 10653377.27. Wang CJ, Wetmore JB, Crary GS, Kasiske BL. The donor kidney biopsy and its implications in predicting graft outcomes: a systematic review. Am J Transplant. 2015; 15:1903–1914. PMID: 25772854.28. Akimoto T, Yoshizawa H, Watanabe Y, Numata A, Yamazaki T, Takeshima E, et al. Characteristics of urinary and serum soluble Klotho protein in patients with different degrees of chronic kidney disease. BMC Nephrol. 2012; 13:155. PMID: 23176706.29. Zou D, Wu W, He Y, Ma S, Gao J. The role of klotho in chronic kidney disease. BMC Nephrol. 2018; 19:285. PMID: 30348110.30. Deng G, Yang A, Wu J, Zhou J, Meng S, Zhu C, et al. The value of older donors' Klotho level in predicting recipients' short-term renal function. Med Sci Monit. 2018; 24:7936–7943. PMID: 30396199.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current Status of Deceased Donor Organ Recovery and Sharing in Korea
- Thrombotic microangiopathy, rare cause of deceased donor acute kidney injury: is a donor biopsy necessary before donation?
- Analysis of donor factors for clinical prediction of recipient after deceased donor renal transplantation in South Korea
- A Preliminary Study to Revise the Marginal Donor Criteria of KONOS in Deceased Donor Kidney Transplantation
- Activation Policy for Brain-dead Organ Donation