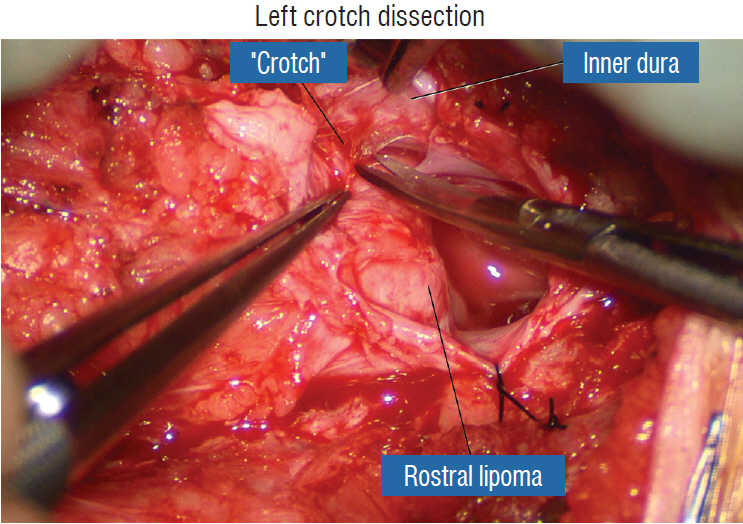
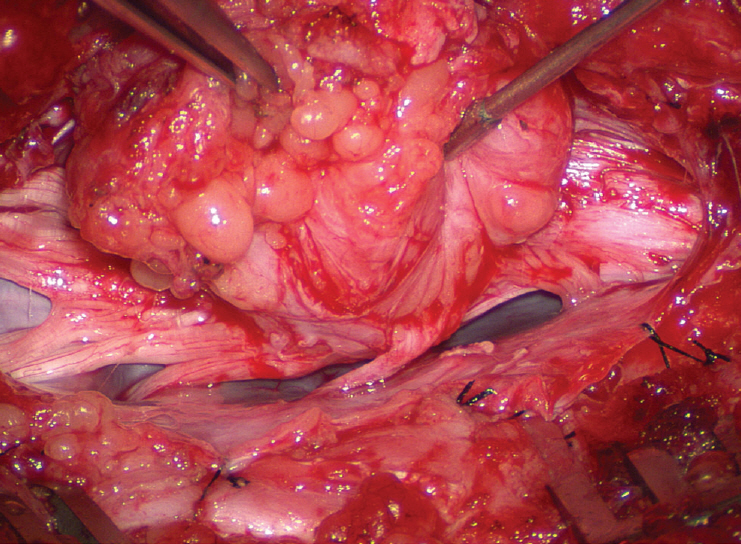
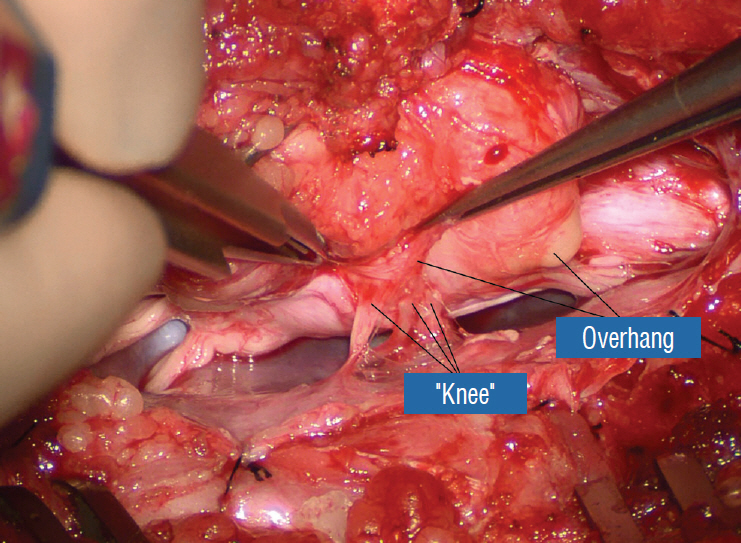
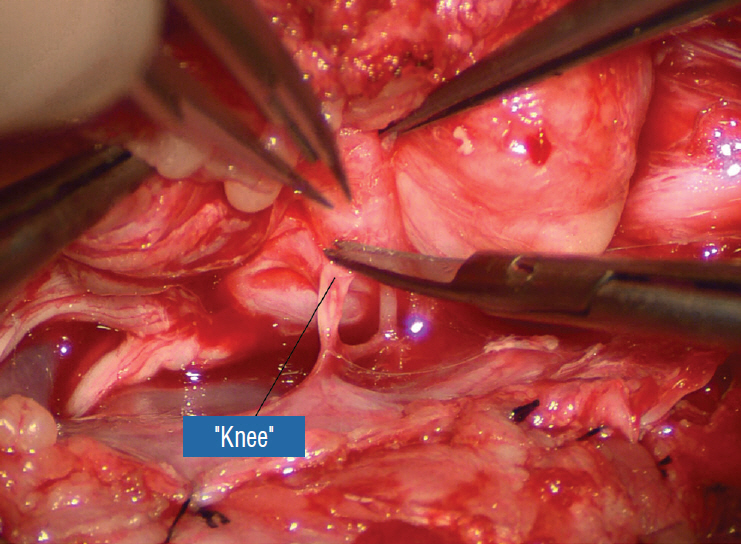
J Korean Neurosurg Soc.
2020 May;63(3):279-313. 10.3340/jkns.2020.0024.
Surgical Management of Complex Spinal Cord Lipomas : A New Perspective
- Affiliations
-
- 1Department of Paediatric Neurosurgery, Great Ormond Street Hospital for Children, NHS Trust, London, UK
- 2Department of Paediatric Neurosurgery, University of California, Davis, CA, USA
- KMID: 2501716
- DOI: http://doi.org/10.3340/jkns.2020.0024
Abstract
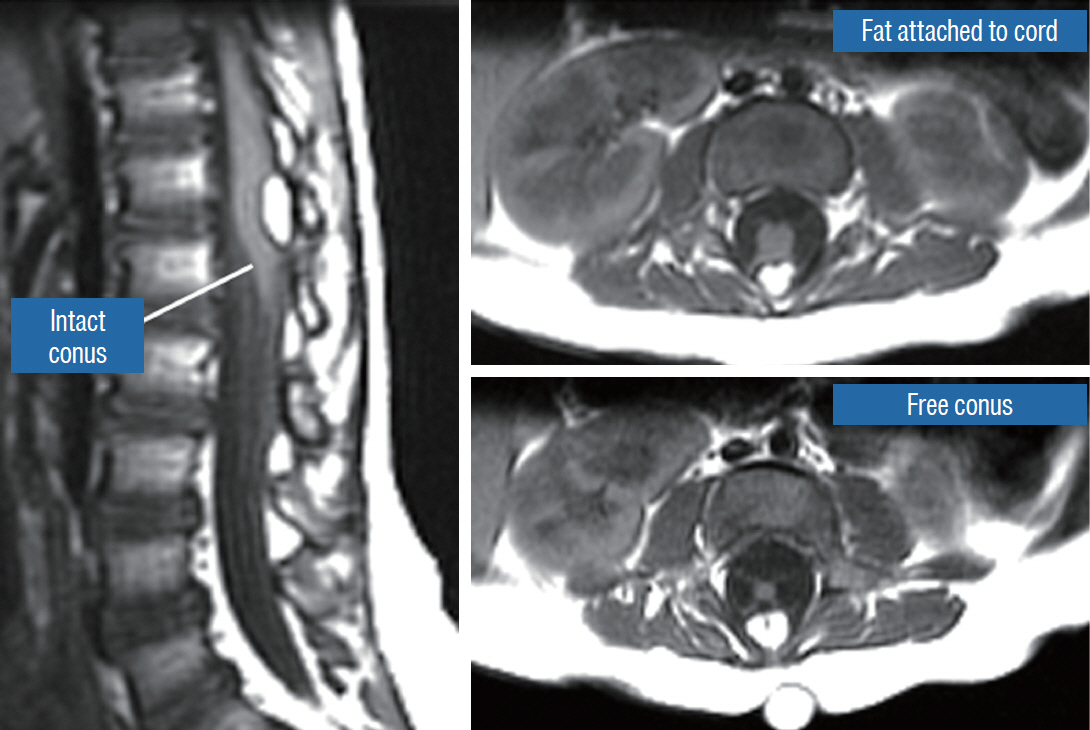
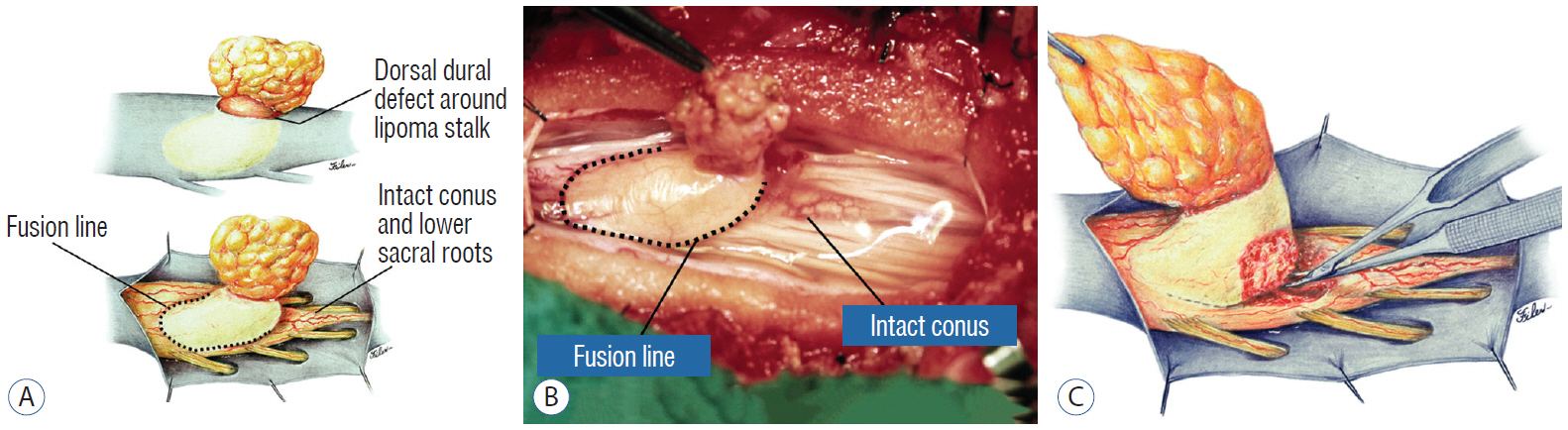
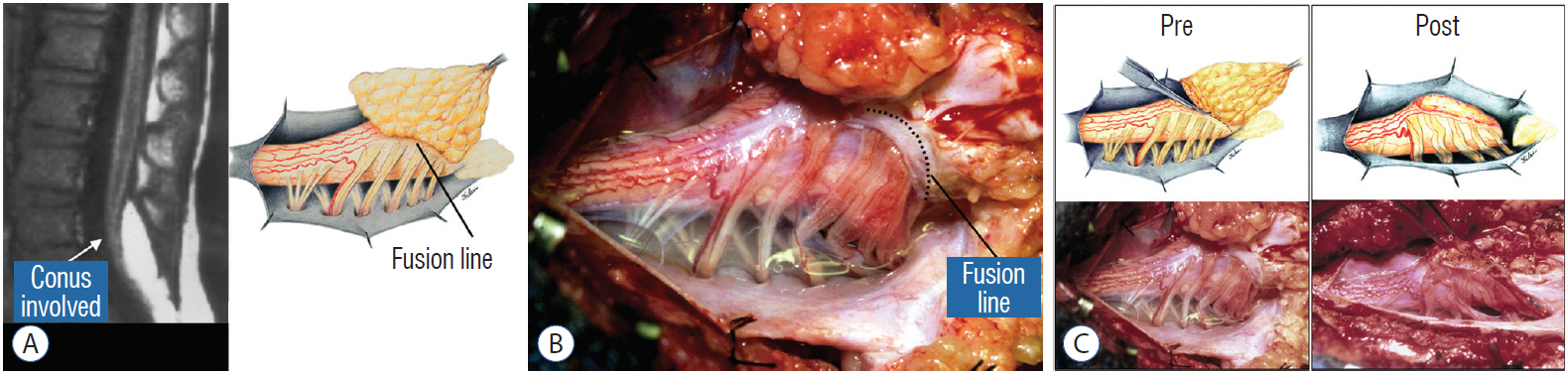
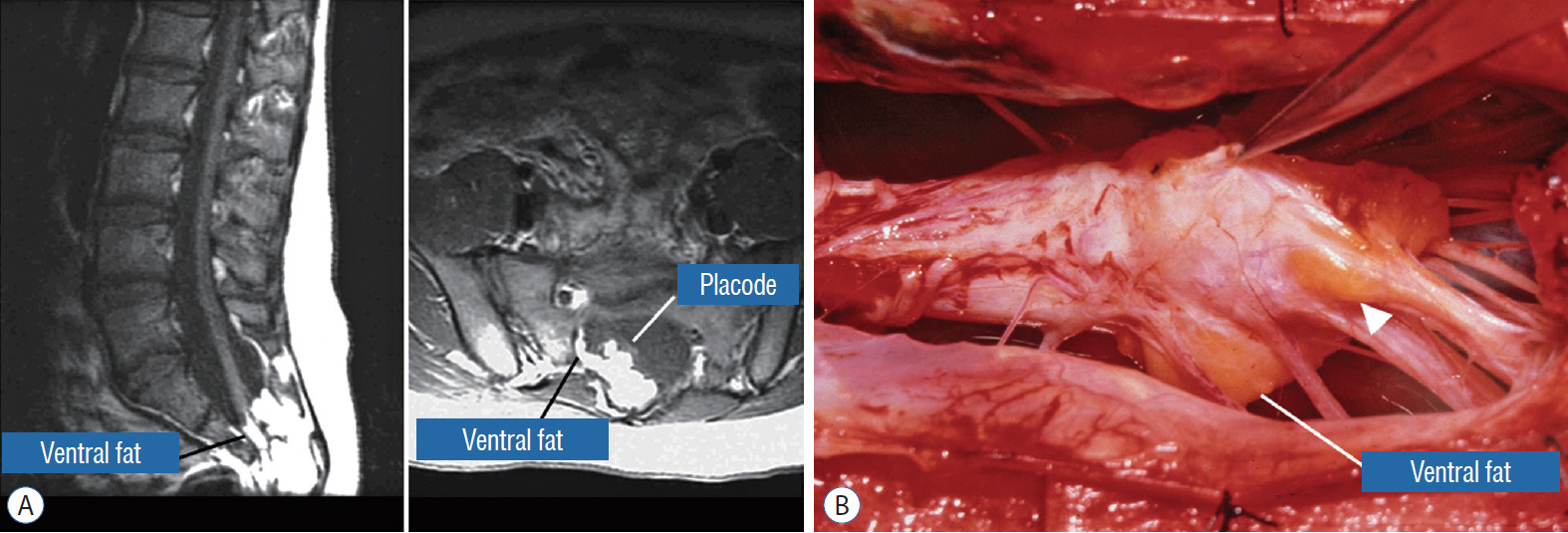
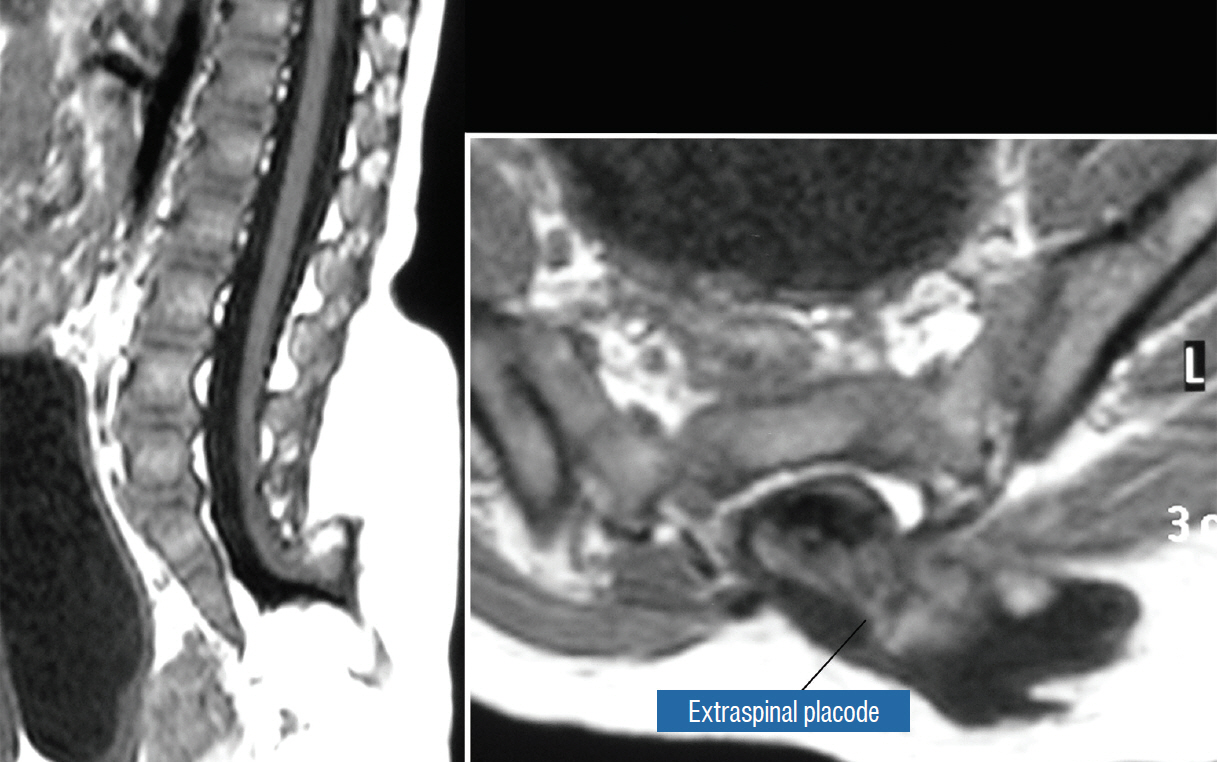
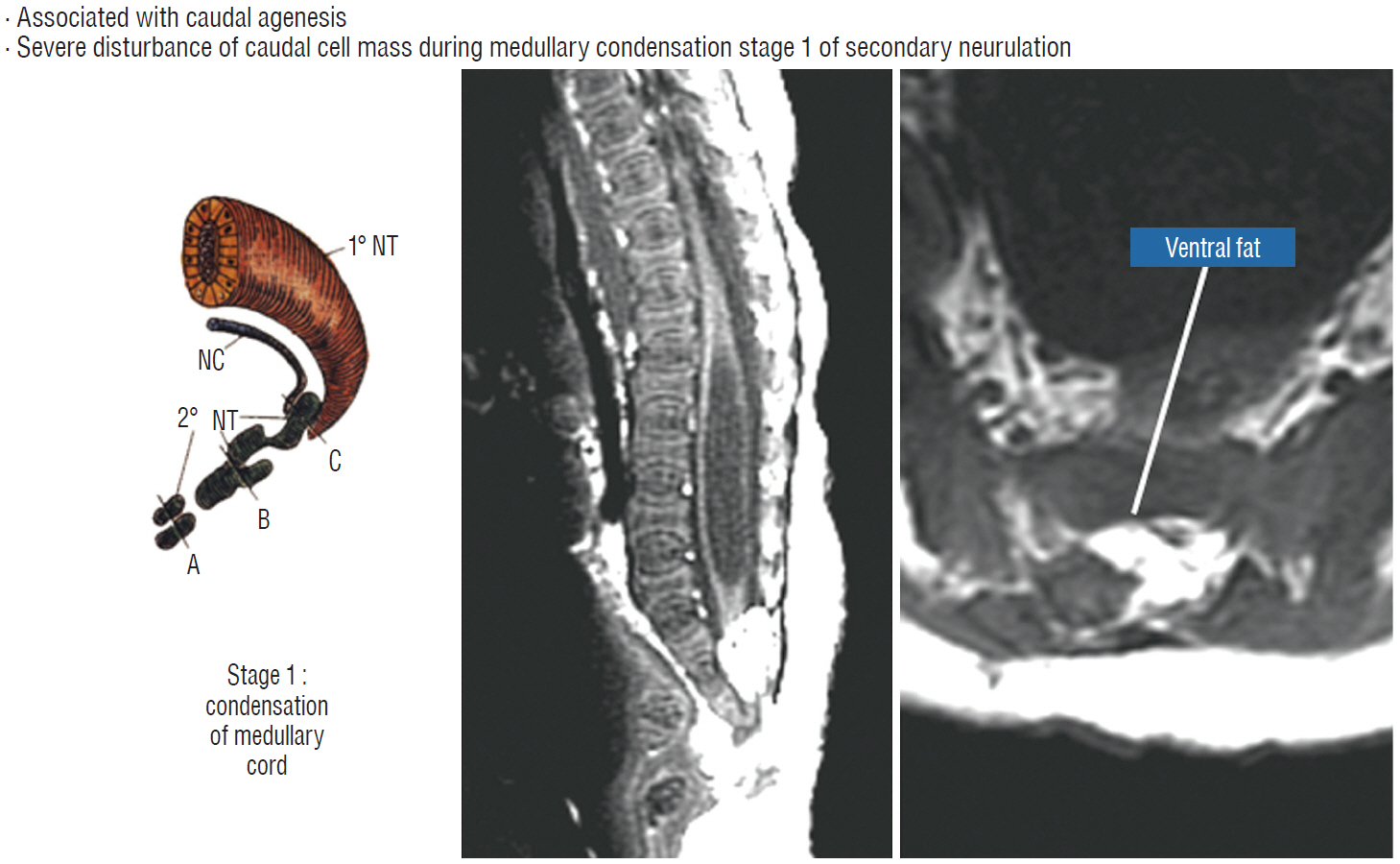
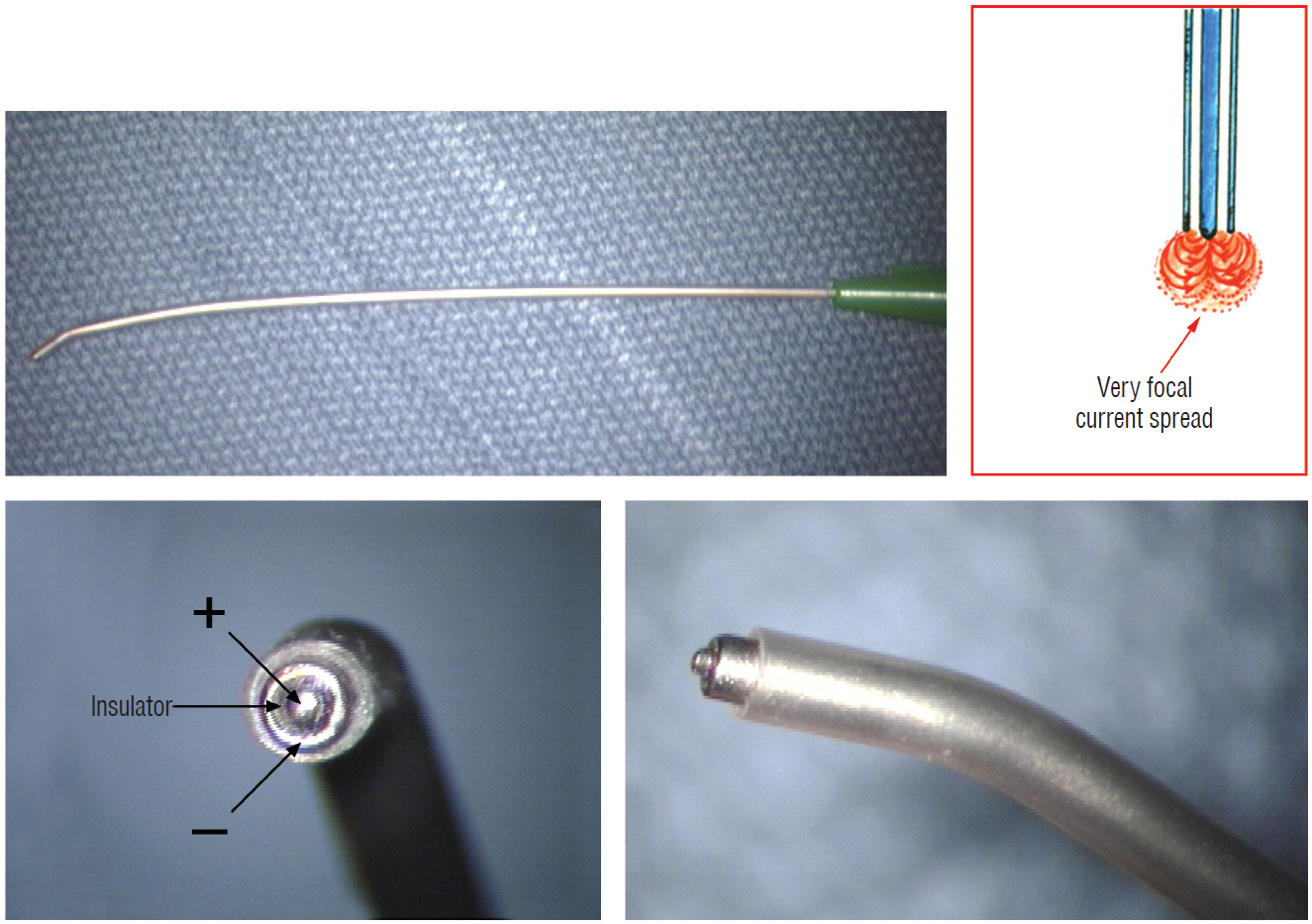
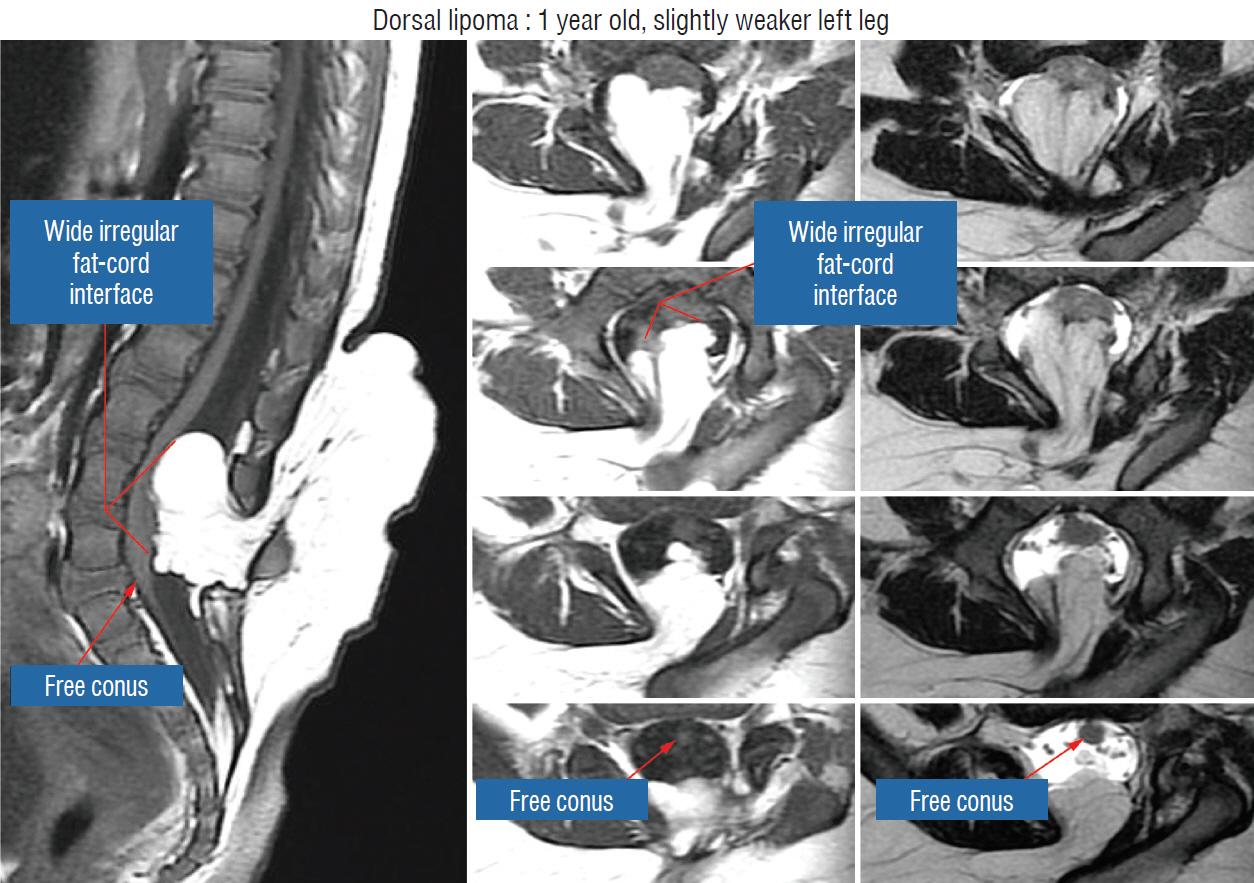
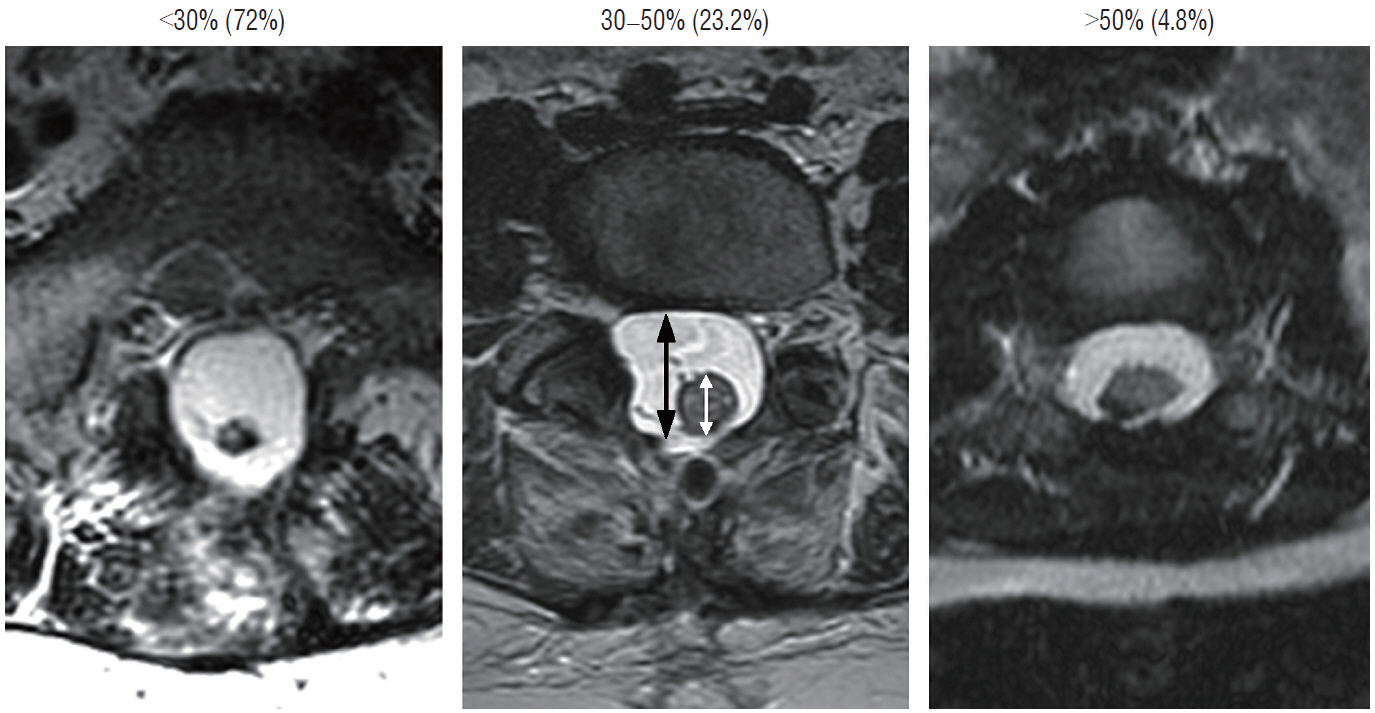
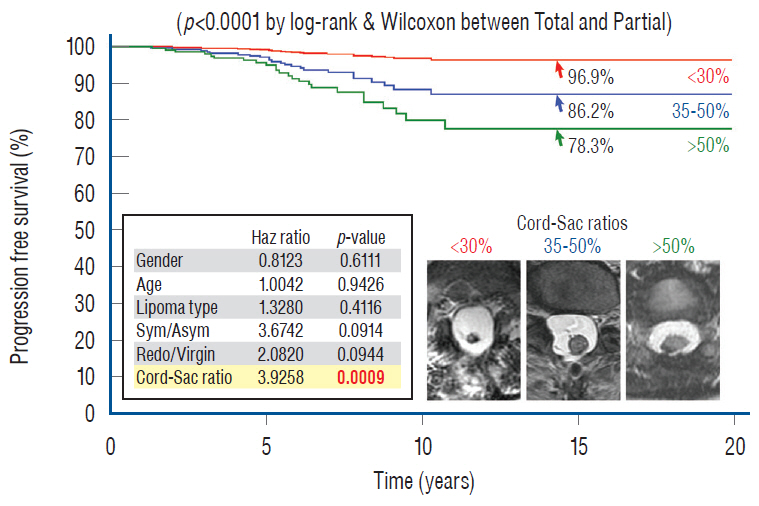
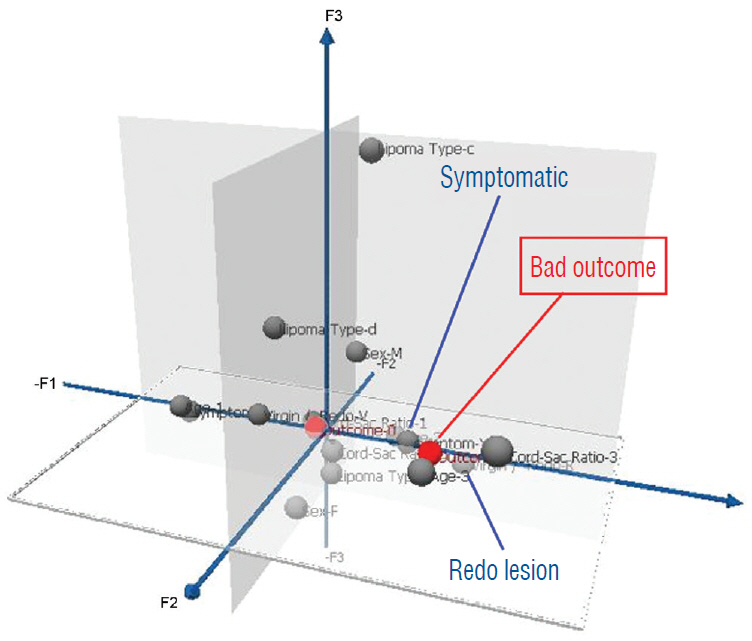
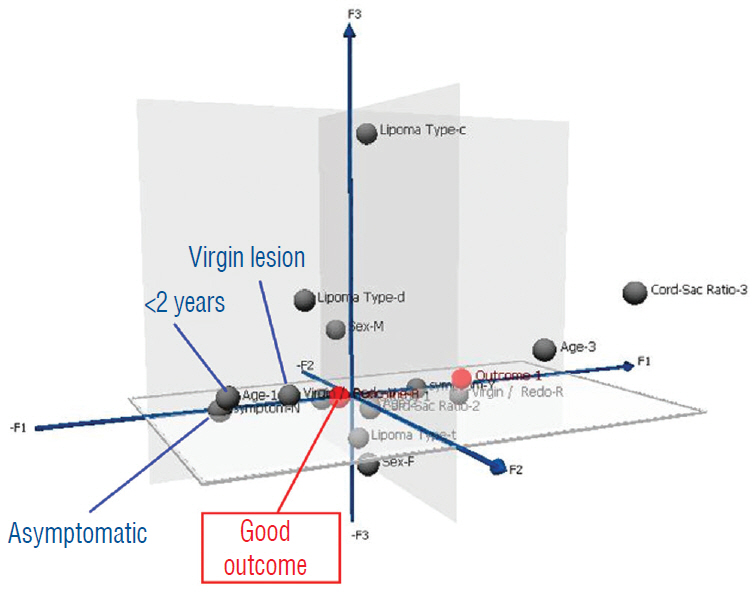
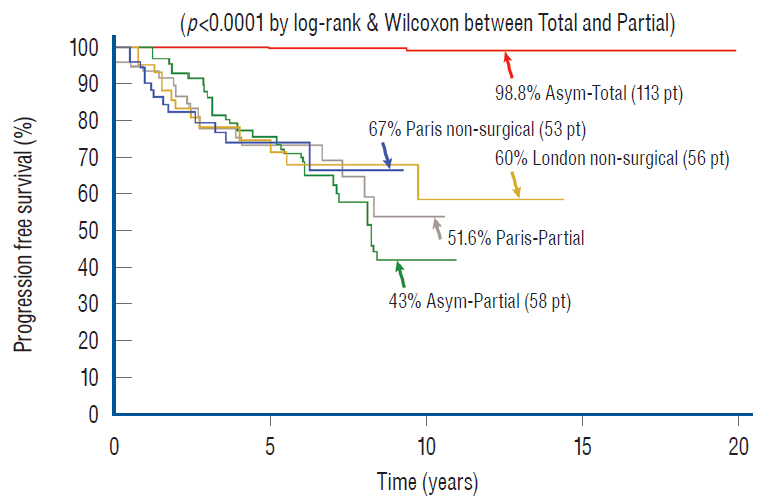
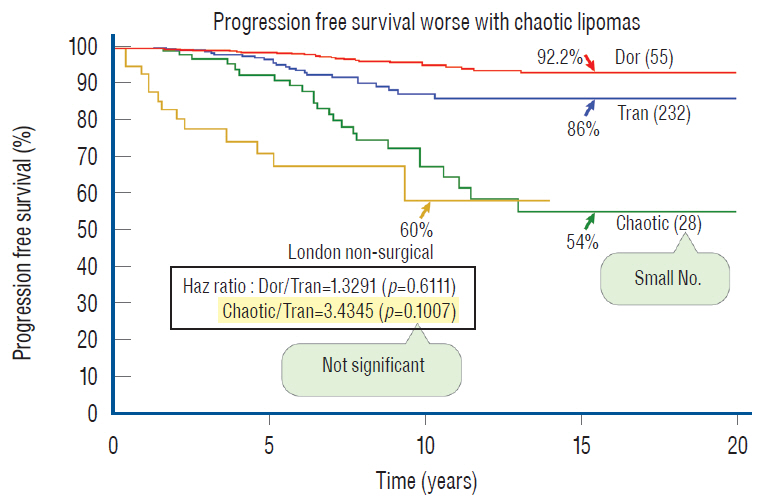
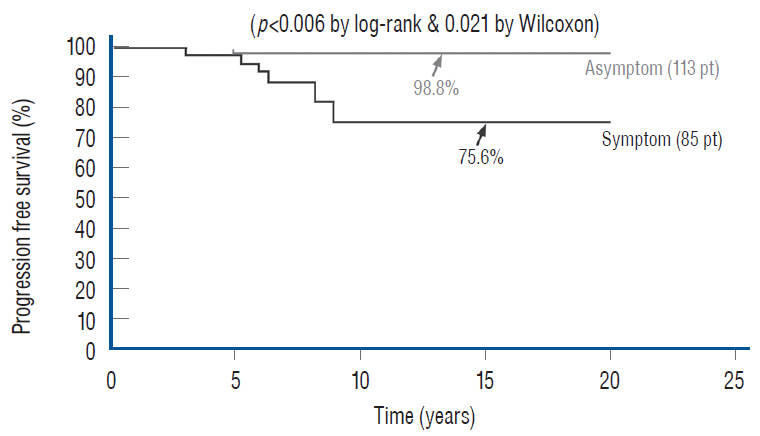
- This review summarises the classification, anatomy and embryogenesis of complex spinal cord lipomas, and describes in some detail the new technique of total lipoma resection and radical reconstruction of the affected neural placode. Its specific mission is to tackle two main issues surrounding the management of complex dysraphic lipomas : whether total resection confers better long term benefits than partial resection, and whether total resection does better than conservative treatment, i.e., no surgery, for asymptomatic lipomas. Accordingly, the 24 years progression-free survival data of the author and colleagues’ series of over 350 cases of total resection are compared with historical data from multiple series (including our own) of partial resection, and total resection data specifically for asymptomatic lesions are compared with the two known series of non-surgical treatment of equivalent patients. These comparisons amply support the author’s recommendation of total resection for most complex lipomas, with or without symptoms. The notable exception is the asymptomatic chaotic lipoma, whose peculiar anatomical relationship with the neural tissue defies even our aggressive surgical approach, and consequently projects worse results (admittedly of small number of cases) than for the other two lipoma subtypes of dorsal and transitional lesions. Prophylactic resection of asymptomatic chaotic lipomas is therefore not currently endorsed.
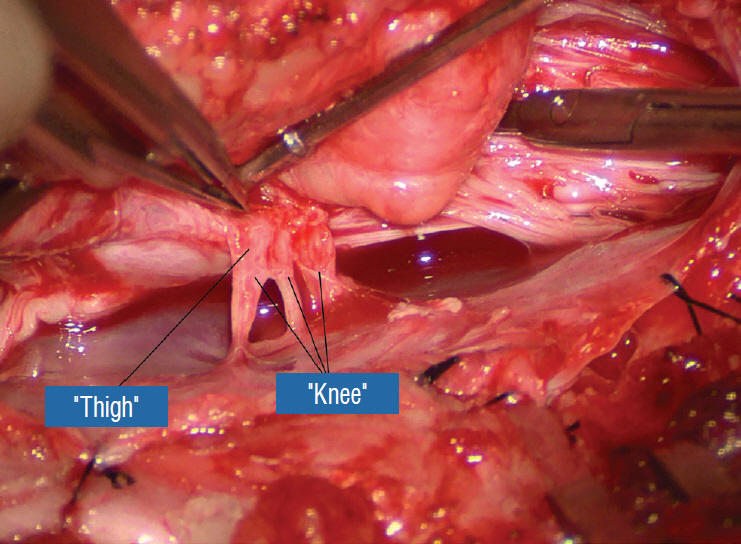
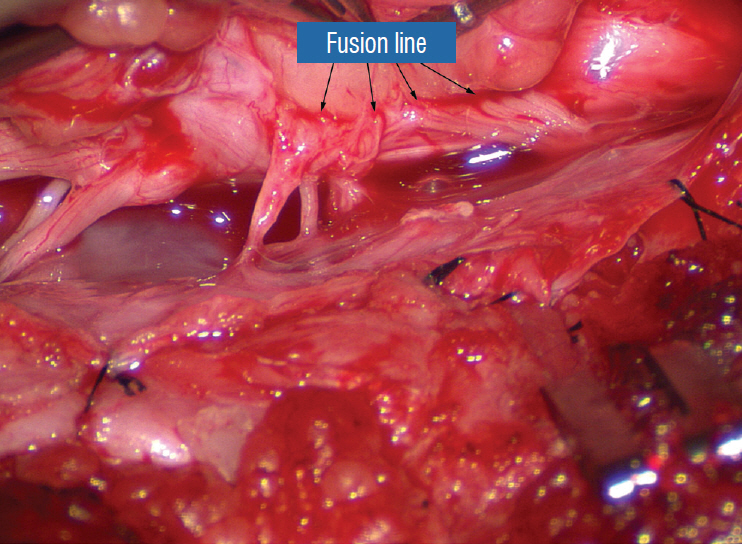
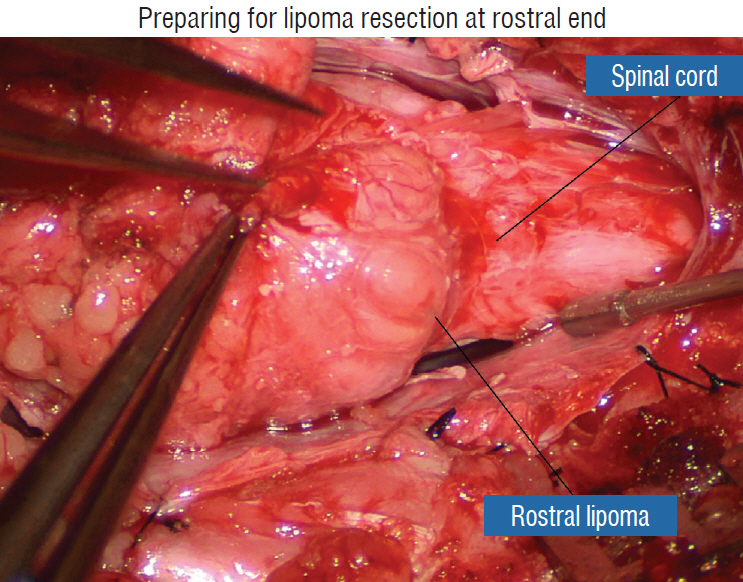
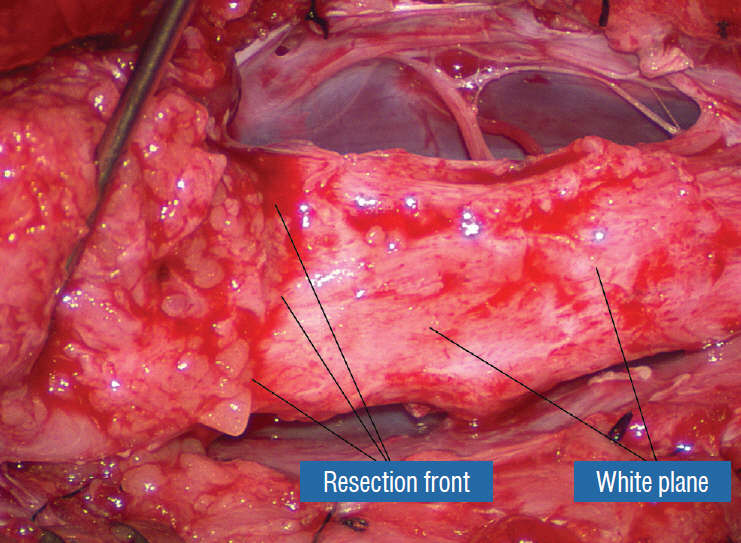
Figure
Reference
-
References
1. Arai H, Sato K, Wachi A. Surgical management in 81 patients with congenital intraspinal lipoma. Child Nerv Syst. 8:171. 1992.2. Atala A, Bauer SB, Dyro FM, Shefner J, Shillito J, Sathi S, et al. Bladder functional changes resulting from lipomyelomeningocele repair. J Urol. 148(2 Pt 2):592–594. 1992.
Article3. Barson AJ. The vertebral level of termination of the spinal cord during normal and abnormal development. J Anat. 106(Pt 3):489–497. 1970.4. Bruce DA, Schut L. Spinal lipomas in infancy and childhood. Childs Brain. 5:192–203. 1979.
Article5. Brunelle F, Sebag G, Baraton J, Carteret M, Martinat P, Pierre-Kahn A. Lumbar spinal cord motion measurement with phase-contrast MR imaging in normal children and in children with spinal lipomas. Pediatr Radiol. 26:265–270. 1996.
Article6. Byrne RW, Hayes EA, Georg TM, McLone DG. Operative resection of 100 spinal lipomas in infants less than 1 year of age. Pediatr Neurosurg. 23:182–186. discussion 186-187. 1995.
Article7. Caldarelli M, McLone DG, Colins JA, Suwa J, Knepper PA. Vitamin A induced neural tube defects in a mouse. Concepts Pediatr Neurosurg. 6:161–171. 1985.8. Chapman PH. Congenital intraspinal lipomas: anatomic considerations and surgical treatment. Childs Brain. 9:37–47. 1982.9. Chapman PH, Davis KR. Surgical treatment of spinal lipomas in childhood. Pediatr Neurosurg. 19:267–275. discussion 274. 1993.
Article10. Cochrane DD, Finley C, Kestle J, Steinbok P. The patterns of late deterioration in patients with transitional lipomyelomeningocele. Eur J Pediatr Surg 10 Suppl. 1:13–17. 2000.
Article11. Colak A, Pollack IF, Albright AL. Recurrent tethering: a common long-term problem after lipomyelomeningocele repair. Pediatr Neurosurg. 29:184–190. 1998.
Article12. Cornette L, Verpoorten C, Lagae L, Plets C, Van Calenbergh F, Casaer P. Closed spinal dysraphism: a review on diagnosis and treatment in infancy. Eur J Paediatr Neurol. 2:179–185. 1998.
Article13. Detwiler SR, Hotzer H. The inductive and formative influence of the spinal cord upon the vertebral column. Bull Hosp Joint Dis. 15:114–123. 1954.14. Dias M, Pang D. Human neural embryogenesis: a description of neural morphogenesis and a review of embryonic mechanisms. In : Pang D, editor. Disorders of the Pediatric Spine. New York: Raven Press;1994.15. Dick EA, de Bruhn R. Ultrasound of the spinal cord in children: its role. Eur Radiol. 13:552–562. 2003.
Article16. Dorward NL, Scatliff JH, Hayward RD. Congenital lumbosacral lipomas: pitfalls in analysing the results of prophylactic surgery. Childs Nerv Syst. 18:326–332. 2002.
Article17. Hamilton HL, Boyd JD, Mossman HM. Human Embryology. ed 4. Baltimore: Williams & Wilkins;1972.18. Hoffman HJ, Hendrick EB, Humphreys RP. The tethered spinal cord: its protean manifestations, diagnosis and surgical correction. Childs Brain. 2:145–155. 1976.
Article19. Hoffman HJ, Taecholarn C, Hendrick EB, Humphreys RP. Management of lipomyelomeningoceles. Experience at the Hospital for Sick Children, Toronto. J Neurosurg. 62:1–8. 1985.20. James CC, Lassman LP. Diastematomyelia and the tight filum terminale. J Neurol Sci. 10:193–196. 1970.
Article21. James HE, Canty TG. Human tails and associated spinal anomalies. Clin Pediatr (Phila). 34:286–288. 1995.
Article22. James HE, Williams J, Brock W, Kaplan GW, U HS. Radical removal of lipomas of the conus and cauda equina with laser microneurosurgery. Neurosurgery. 13:340–345. 1984.
Article23. Jones PH, Love JG. Tight filum terminale. AMA Arch Surg. 73:556–566. 1956.
Article24. Källén B. Early embryogenesis of the central nervous system with special reference to closure defects. Dev Med Child Neurol. 10(Suppl 6):44–53. 1968.25. Kanev PM, Lemire RJ, Loeser JD, Berger MS. Management and long-term follow-up review of children with lipomyelomeningocele, 1952-1987. J Neurosurg. 74:48–52. 1990.
Article26. Koyanagi I, Iwasaki Y, Hida K, Abe H, Isu T, Akino M. Surgical treatment supposed natural history of the tethered cord with occult spinal dysraphism. Childs Nerv Syst. 13:268–274. 1997.
Article27. Kulkarni AV, Pierre-Kahn A, Zerah M. Conservative Management of asymptomatic spinal lipomas of the conus. Neurosurgery. 54:868–873. discussion 873-875. 2004.
Article28. Kunitomo K. The development and reduction of the tail and of the caudal end of the spinal cord. Carnegie Contr Embryol. 8:161–203. 1918.29. La Marca F, Grant JA, Tomita T, McLone DG. Spinal lipomas in children: Outcome of 270 procedures. Pediatr Neurosurg. 26:8–16. 1997.
Article30. Marin-Padilla M. Clinical and experimental rachischisis. In : Vinken PS, Bruyn GW, editors. Handbook of clinical neurology. Amsterdam: North-Holland;1978. 32:p. 159–191.31. Marin-Padilla M. Mesodermal alterations induced by hypervitaminosis A. J Embryol Exp Morphol. 15:261–269. 1966.
Article32. Marin-Padilla M. Morphogenesis of anencephaly and related malformations in Current topics in pathology. Heidelberg: Springer;1921. p. 145–174.33. Marin-Padilla M. Morphogenesis of experimental encephalocele (Cranioschisis occulta). J Neurol Sci. 46:83–99. 1980.
Article34. Marin-Padilla M. Notochordal-basichondrocranium relationships: abnormalities in experimental axial skeletal (dysraphic) disorders. J Embryol Exp Morphol. 53:15–38. 1979.
Article35. Marin-Padilla M. The tethered cord syndrome: developmental considerations. In : Holtzmann RNN, Stein BM, editors. The tethered spinal cord. New York: Thieme;1985. p. 3–13.36. Marin-Padilla M, Marin-Padilla TM. Morphogenesis of experimentally induced Arnold--Chiari malformation. J Neurol Sci. 50:29–55. 1981.
Article37. McGuire EJ, Woodside JR, Borden TA, Weiss RM. Prognostic value of urodynamic testing in myelodysplastic patients. J Urol. 126:205–209. 1981.
Article38. McGuire EJ. The innervation and function of the lower urinary tract. J Neurosurg. 65:278–285. 1986.
Article39. McLone DG, Knepper PA. Role of complex carbohydrates and neurulation. Pediatr Neurosci. 1(2-9):1985–1986.
Article40. McLone DG, Mutluer S, Naidich TP. Lipomeningoceles of the conus medullaris. In : Karger S, editor. Concepts in Pediatric Neurosurgery. Basel: Karger;1982. p. 171–177.41. McLone DG, Naidich TP. Laser resection of fifty spinal lipomas. Neurosurgery. 18:611–615. 1986.
Article42. McLone DG, Naidich TP. Spinal dysraphism: Experimental and clinical. In : Holtzman RN, Stein BM, editors. The Tethered Spinal Cord. New York: Thieme-Stratton;1985.43. McLone DG, Suwa J, Collins JA, Poznaski S, Knepper PA. Neurulation: biochemical and morphological studies on primary and secondary neural tube defects. Concepts Pediatr Neurosurg. 4:15–29. 1983.44. Morriss-Kay GM, Crutch B. Culture of rat embryos with beta-D-xyloside: evidence of a role for proteoglycans in neurulation. J Anat. 134(Pt 3):491–506. 1982.45. Müller F, O’Rahilly R. The development of the human brain, the closure of the caudal neuropore, and the beginning of secondary neurulation at Stage 12. Anat Embryol (Berl). 176:413–430. 1987.
Article46. O’Rahilly R, Meyer DB. The timing and sequence of events in the development of the human vertebral column during the embryonic period proper. Anat Embryol (Berl). 157:167–176. 1979.
Article47. O’Shea KS, Kaufman MH. Phospholipase C-induced neural tube defects in the mouse embryo. Experientia. 36:1217–1219. 1980.
Article48. Pang D. Commentary to the article: asymptomatic lumbosacral lipomas- -a natural history study, by Wykes V, Desai D, and Thompson DNP. Childs Nerv Syst. 28:1741–1742. 2012.
Article49. Pang D. Electrophysiological monitoring for tethered cord surgery. In : Yamada S, editor. Tethered cord syndrome. ed 2. New York: Thieme Medical Publishers;2010. p. 199–209.50. Pang D. Intraoperative neurophysiology of the conus medullaris and cauda equina. Childs Nerv Syst. 26:411–412. 2010.
Article51. Pang D. Spinal Cord Lipoma. In : Batjer H, Loftus C, editors. Textbook of Neurological Surgery. New Jersey: Lippincott, Williams and Wilkins;2002.52. Pang D. Spinal Cord Lipomas. In : Pang D, editor. Disorders of the Pediatric Spine. New York: Raven Press;1995. p. 175–201.53. Pang D. Surgical management of complex spinal cord lipomas: how, why, and when to operate. A review. J Neurosurg Pediatr. 23:537–556. 2019.
Article54. Pang D. Tethered Cord Syndrome. In : Hoffman HJ, editor. Advances in Pediatric Neurosurgery. Philadelphia: Hanley and Belfus, Inc;1986. p. 45–79.55. Pang D. Total resection of complex spinal cord lipomas: how, why, and when to operate? Neurol Med Chir (Tokyo). 55:695–721. 2015.
Article56. Pang D, Casey K. Use of an anal sphincter pressure monitor during operations on the sacral spinal cord and nerve roots. Neurosurgery. 13:562–568. 1983.
Article57. Pang D, Wilberger JE Jr. Tethered cord syndrome in adults. J Neurosurg. 57:32–47. 1982.
Article58. Pang D, Zovickian J, Moes GS. Retained medullary cord in humans: late arrest of secondary neurulation. Neurosurgery. 68:1500–1519. discussion 1519. 2011.
Article59. Pang D, Zovickian J, Oviedo A. Long-term outcome of total and near-total resection of spinal cord lipomas and radical reconstruction of the neural placode: part I-surgical technique. Neurosurgery. 65:511–528. discussion 528-529. 2009.60. Pang D, Zovickian J, Oviedo A. Long-term outcome of total and near-total resection of spinal cord lipomas and radical reconstruction of the neural placode, part II: outcome analysis and preoperative profiling. Neurosurgery. 66:253–272. discussion 272-273. 2010.61. Pang D, Zovickian J, Wong ST, Hou YJ, Moes GS. Surgical treatment of complex spinal cord lipomas. Childs Nerv Syst. 29:1485–1513. 2013.
Article62. Pierre-Kahn A, Lacombe J, Pichon J, Giudicelli Y, Renier D, Sainte-Rose C, et al. Intraspinal lipomas with spina bifida. Prognosis and treatment in 73 cases. J Neurosurg. 65:756–761. 1986.63. Pierre-Kahn A, Zerah M, Renier D, Cinalli G, Sainte-Rose C, Lellouch-Tubiana A, et al. Congenital lumbosacral lipomas. Childs Nerv Syst. 13:298–334. discussion 335. 1997.
Article64. Sathi S, Madsen JR, Bauer S, Scott RM. Effect of surgical repair on the neurologic function in infants with lipomeningocele. Pediatr Neurosurg. 19:256–259. 1993.
Article65. Schoenwolf GC. Histological and ultrastructural observations of tail bud formation in the chick embryo. Anat Rec. 193:131–147. 1979.
Article66. Schoenwolf GC. Histological and ultrastructural studies of secondary neurulation in mouse embryos. Am J Anat. 169:361–376. 1984.
Article67. Schut L, Bruce DA, Sutton LN. The management of the child with a lipomyelomeningocele. Child Neurosurg. 30:464–476. 1983.
Article68. Stolke D, Zumkeller M, Seifert V. Intraspinal lipomas in infancy and childhood causing a tethered cord syndrome. Neurosurg Rev. 11:59–65. 1988.
Article69. Streeter GL. Factors involved in the formation of the filum terminale. Am J Anat. 25:1–11. 1919.
Article70. Sutton LN. Lipomyelomeningocele. Neurosurg Clin N Am. 6:325–338. 1995.
Article71. Talwalker VC, Datsur DK. Ectopic spinal cord myelomeningocele with tethering: a clinicopathological entity. Dev Med Child Neurol. 16(Suppl 32):159–160. 1974.72. Toole BP. Glycosaminoglycans in morphogenesis. In : Hay E, editor. Cell Biology of Extracellular Matrix. New York: Plenum Press;1981. p. 229–294.73. Wykes V, Desai D, Thompson DN. Asymptomatic lumbosacral lipomas- -a natural history study. Childs Nerv Syst. 28:1731–1739. 2012.
Article74. Xenos C, Sgouros S, Walsh R, Hockley A. Spinal lipomas in children. Pediatr Neurosurg. 32:295–307. 2000.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Intramedullary Spinal Cord Lipoma without Spinal Dysraphism
- Long Term Outcome of Non-Dysraphic Intramedullary Spinal Cord Lipomas in Adults: Case Series and Review
- Spinal Intramedullary Lipoma without Dysraphism
- A Case of Intradural Spinal Lipoma
- Perspectives on Spinal Dysraphism : Past, Present, and Future