Ewha Med J.
2020 Apr;43(2):35-38. 10.12771/emj.2020.43.2.35.
Severe Acute Kidney Injury with Familial Renal Hypouricemia Confirmed by Genotyping of SLC22A12
- Affiliations
-
- 1Department of Pediatrics, Ewha Womans University School of Medicine
- 2Seoul National University Children’s Hospital, Seoul, Korea
- KMID: 2501246
- DOI: http://doi.org/10.12771/emj.2020.43.2.35
Abstract
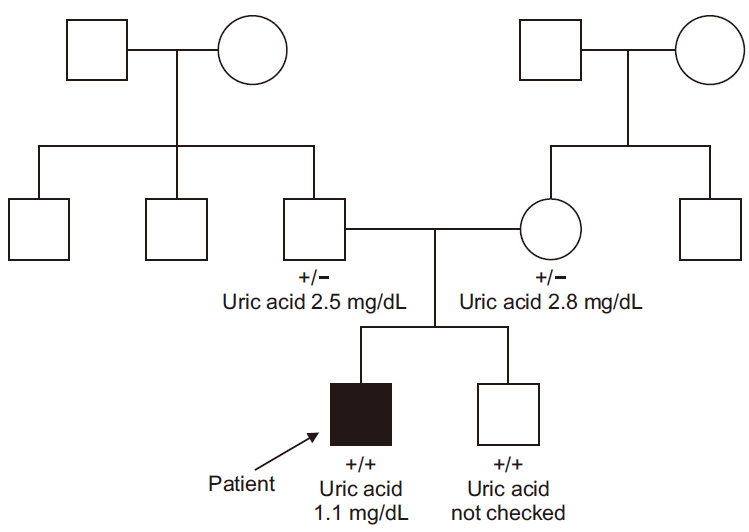
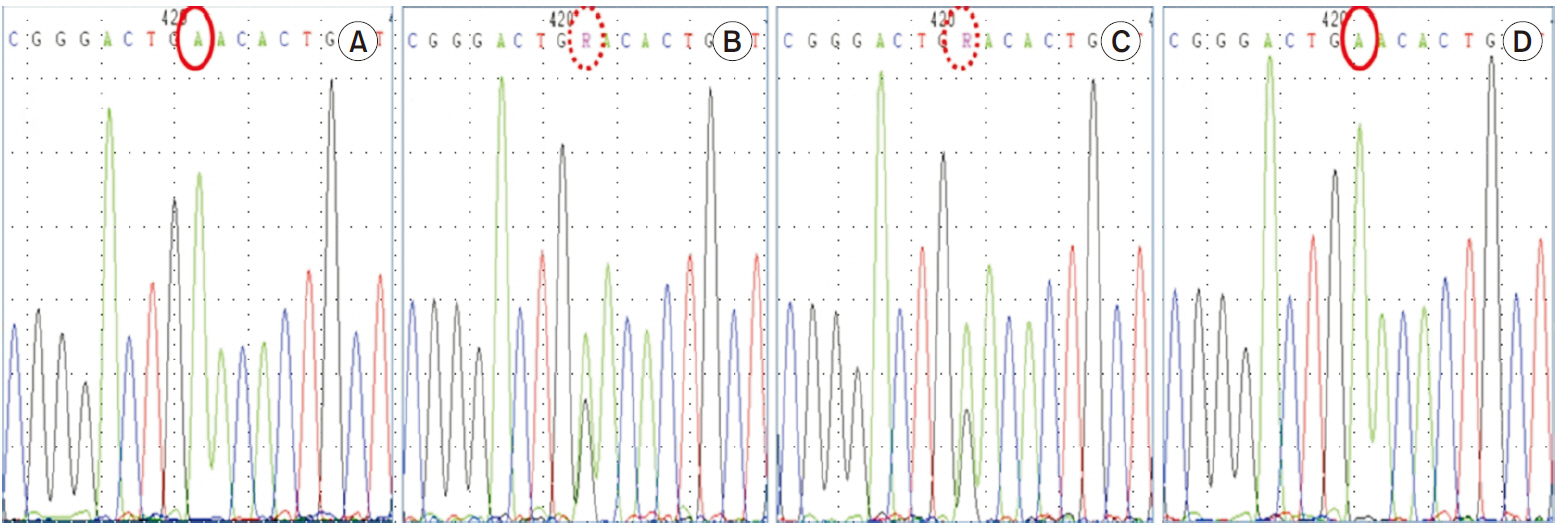
- Idiopathic renal hypouricemia is a hereditary disease characterized by abnormally high renal uric acid clearance. A defect in the SLC22A12 genes, which encodes the renal uric acid transporter, URAT1, is the known major causes of this disorder. Most patients are clinically silent, but exercise-induced acute kidney injury, urolithiasis or hematuria may develop. The patient presented with azotemia, decreased urine output and abdominal pain without vigorous exercise past history. He was diagnosed with rapidly progressive glomerulonephritis at admission, but low serum uric acid level was persisted. Since the diagnosis of the patient was familial renal hypouricemia, we performed sequence analysis of the SLC22A12 gene in all family members. We report a case of 17-year-old boy with severe acute kidney injury with familial renal hypouricemia confirmed by genotyping of SLC22A12 .
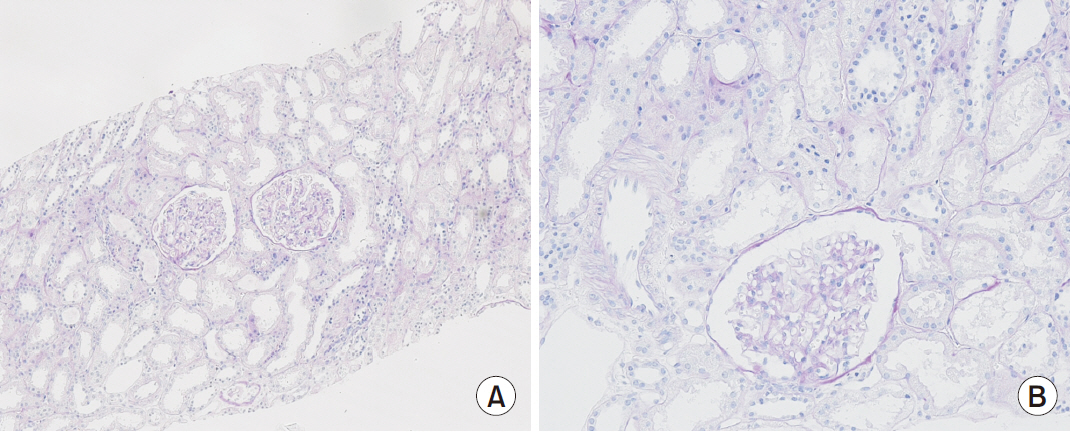
Figure
Reference
-
1. Enomoto A, Kimura H, Chairoungdua A, Shigeta Y, Jutabha P, Cha SH, et al. 2002; Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature. 417:447–452. DOI: 10.1038/nature742. PMID: 12024214.2. Stiburkova B, Sebesta I, Ichida K, Nakamura M, Hulkova H, Krylov V, et al. 2013; Novel allelic variants and evidence for a prevalent mutation in URAT1 causing renal hypouricemia: biochemical, genetics and functional analysis. Eur J Hum Genet. 21:1067–1073. DOI: 10.1038/ejhg.2013.3. PMID: 23386035. PMCID: PMC3778361.
Article3. Vitart V, Rudan I, Hayward C, Gray NK, Floyd J, Palmer CN, et al. 2008; SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout. Nat Genet. 40:437–442. DOI: 10.1038/ng.106. PMID: 18327257.
Article4. Ohta T, Sakano T, Ogawa T, Kato J, Awaya Y, Kihara H, et al. 2002; Exercise-induced acute renal failure with renal hypouricemia: a case report and a review of the literature. Clin Nephrol. 58:313–316. DOI: 10.5414/CNP58313. PMID: 12400848.
Article5. Hirasaki S, Koide N, Fujita K, Ogawa H, Tsuji T. 1997; Two cases of renal hypouricemia with nephrolithiasis. Intern Med. 36:201–205. DOI: 10.2169/internalmedicine.36.201. PMID: 9144014.
Article6. Hisatome I, Tanaka Y, Ogino K, Shimoyama M, Hiroe K, Tsuboi M, et al. 1998; Hematuria in patients with renal hypouricemia. Intern Med. 37:40–46. DOI: 10.2169/internalmedicine.37.40. PMID: 9510398.
Article7. Sebesta I, Stiburkova B, Bartl J, Ichida K, Hosoyamada M, Taylor J, et al. 2011; Diagnostic tests for primary renal hypouricemia. Nucleosides Nucleotides Nucleic Acids. 30:1112–1116. DOI: 10.1080/15257770.2011.611483. PMID: 22132965.
Article8. Akaoka I, Nishizawa T, Yano E, Takeuchi A, Nishida Y. 1975; Familial hypouricaemia due to renal tubular defect of urate transport. Ann Clin Res. 7:318–324.9. Cheong HI, Kang JH, Lee JH, Ha IS, Kim S, Komoda F, et al. 2005; Mutational analysis of idiopathic renal hypouricemia in Korea. Pediatr Nephrol. 20:886–890. DOI: 10.1007/s00467-005-1863-3. PMID: 15912381.
Article10. Komoda F, Sekine T, Inatomi J, Enomoto A, Endou H, Ota T, et al. 2004; The W258X mutation in SLC22A12 is the predominant cause of Japanese renal hypouricemia. Pediatr Nephrol. 19:728–733. DOI: 10.1007/s00467-004-1424-1. PMID: 15054642.
Article11. Kim HO, Ihm CG, Jeong KH, Kang HJ, Kim JM, Lim HS, et al. 2015; A case report of familial renal hypouricemia confirmed by genotyping of SLC22A12, and a literature review. Electrolyte Blood Press. 13:52–57. DOI: 10.5049/EBP.2015.13.2.52. PMID: 26848304. PMCID: PMC4737662.12. Ochi A, Takei T, Ichikawa A, Kojima C, Moriyama T, Itabashi M, et al. 2012; A case of acute renal failure after exercise with renal hypouricemia demonstrated compound heterozygous mutations of uric acid transporter 1. Clin Exp Nephrol. 16:316–319. DOI: 10.1007/s10157-011-0557-3. PMID: 22045201.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case Report of Familial Renal Hypouricemia Confirmed by Genotyping of SLC22A12, and a Literature Review
- A Case of Idiopathic Renal Hypouricemia with URAT1 Gene Mutation who Showed Persistent Orange-colored Urine
- A Case of Idiopathic Renal Hypouricemia with SLC22A12 Gene Mutation Showing General Weakness and Incidental Renal Stone
- A case of idiopathic renal hypouricemia
- A Case of Exercise-induced Acute Renal Failure with G774A Mutation in SCL22A12 Causing Renal Hypouricemia