Cancer Res Treat.
2020 Jan;52(1):218-245. 10.4143/crt.2019.217.
Anterior Gradient 3 Promotes Breast Cancer Development and Chemotherapy Response
- Affiliations
-
- 1Department of Tumor Cell Biology, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer, Tianjin, China
- 2Tianjin’s Clinical Research Center for Cancer, Tianjin, China
- 3Key Laboratory of Cancer Prevention and Therapy, Tianjin, Chin
- 4Key Laboratory of Breast Cancer Prevention and Therapy, Tianjin Medical University, Ministry of Education, Tianjin, China
- 5Department of Breast Cancer Pathology and Research Laboratory, Tianjin Medical University Cancer Institute and Hospital, Tianjin, China
- KMID: 2501215
- DOI: http://doi.org/10.4143/crt.2019.217
Abstract
- Purpose
Anterior gradient 3 (AGR3) belongs to human anterior gradient (AGR) family. The function of AGR3 on cancer remains unknown. This research aimed to investigate if AGR3 had prognostic values in invasive ductal carcinoma (IDC) of breast cancer and could promote tumor progression.
Materials and Methods
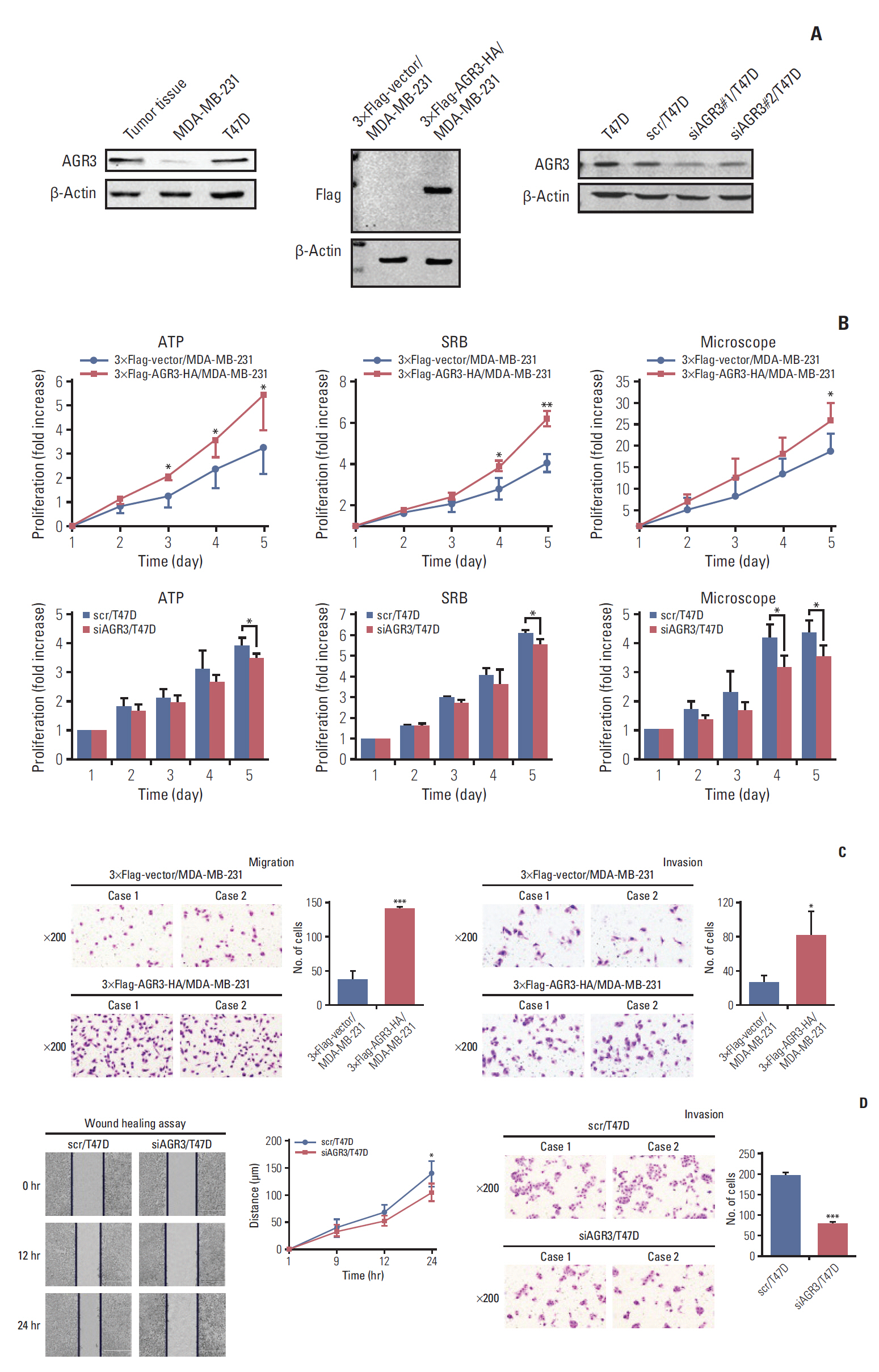
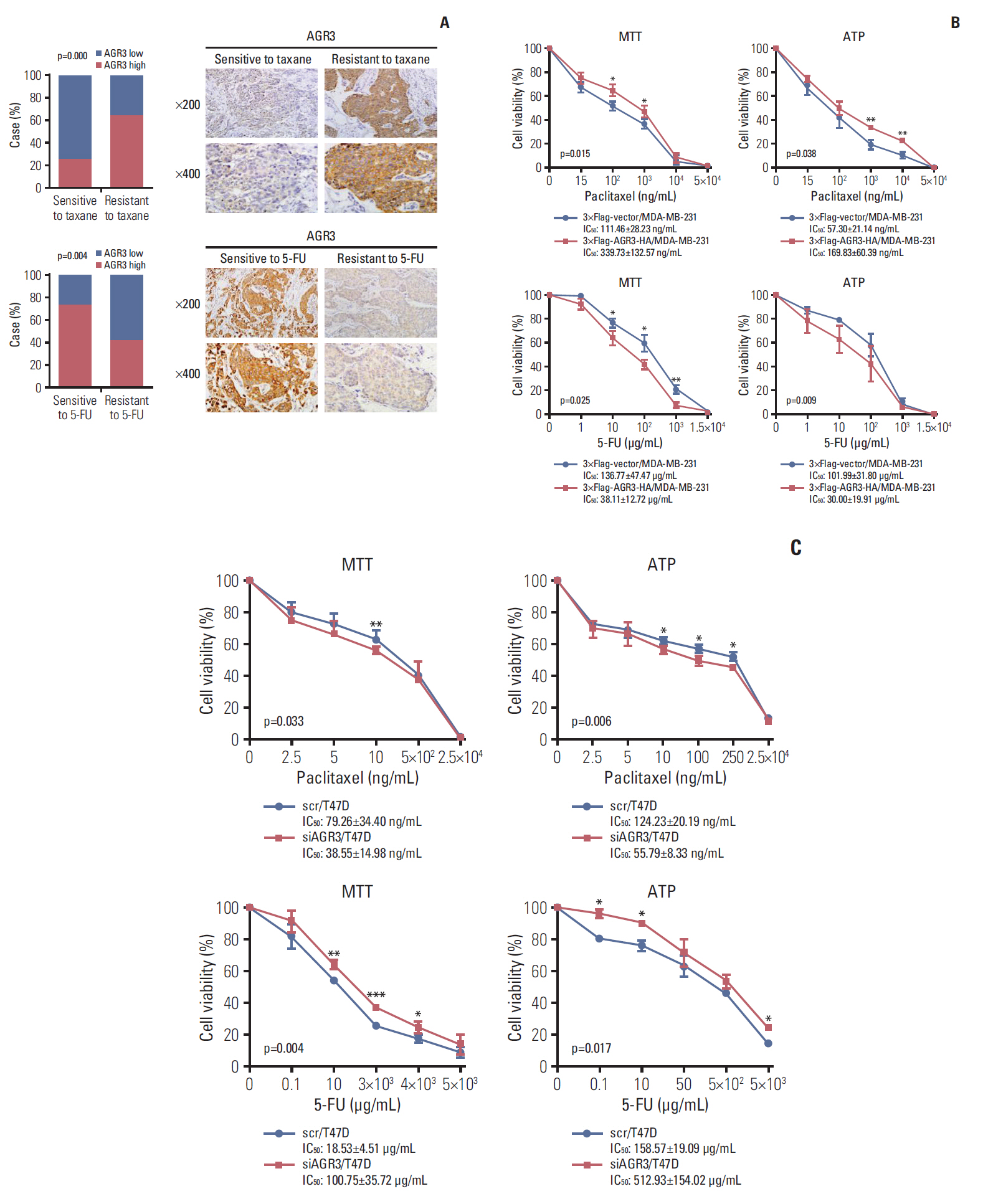
AGR3 expression was detected in breast benign lesions, ductal carcinoma in situ and IDC by immunohistochemistry analysis. AGR3’s correlations with clinicopathological features and prognosis of IDC patients were analyzed. By cell function experiments, collagen gel droplet-embedded culture drug sensitivity test and cytotoxic analysis, AGR3’s impacts on proliferation, invasion ability, and chemotherapeutic drug sensitivity of breast cancer cells were also detected.
Results
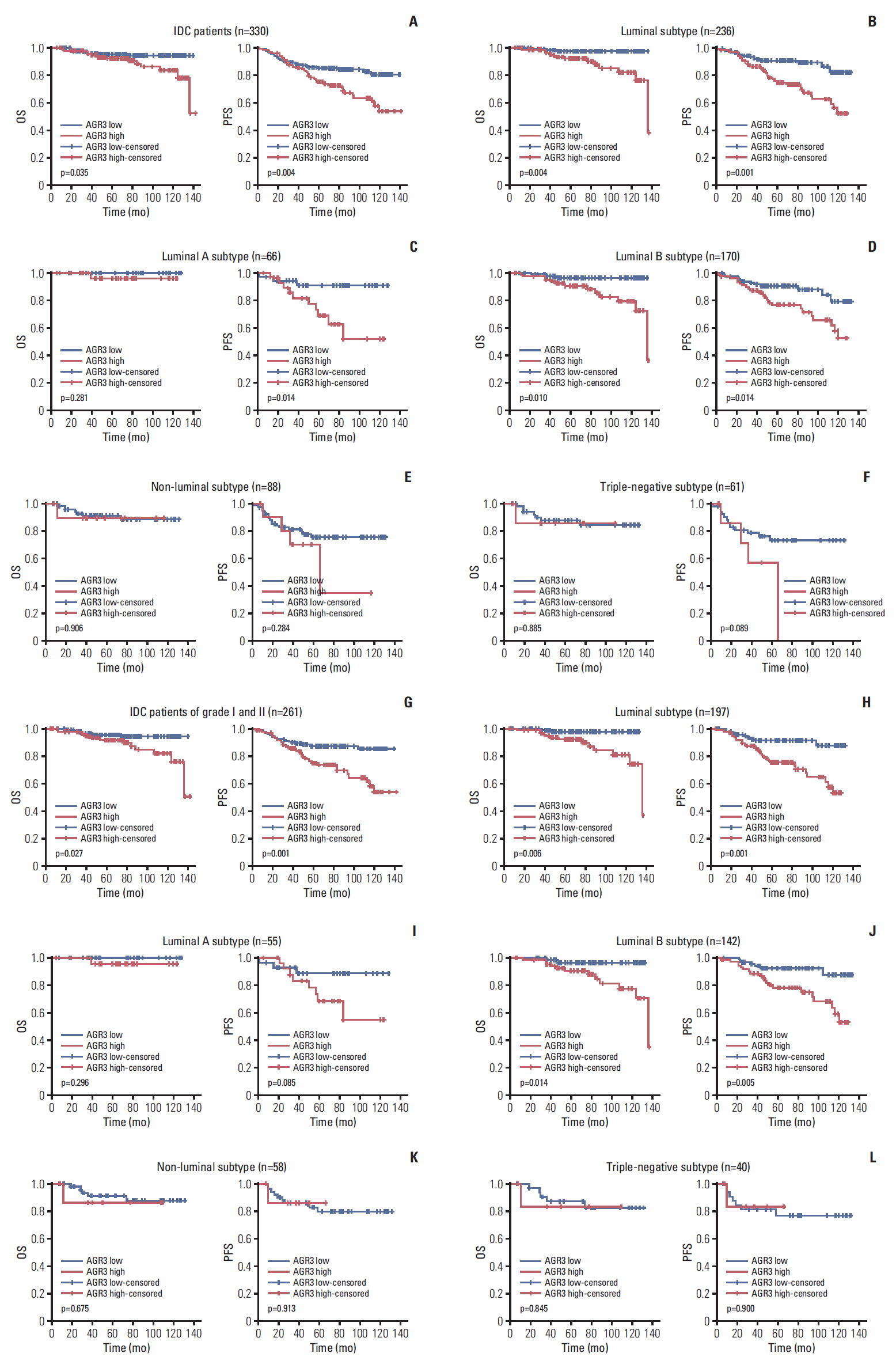
AGR3 was up-regulated in luminal subtype of histological grade I-II of IDC patients and positively correlated with high risks of recurrence and distant metastasis. AGR3 high expression could lead to bone or liver metastasis and predict poor prognosis of luminal B. In cell lines, AGR3 could promote proliferation and invasion ability of breast cancer cells which were consistent with clinical analysis. Besides, AGR3 could indicate poor prognosis of breast cancer patients treated with taxane but a favorable prognosis with 5-fluoropyrimidines. And breast cancer cells with AGR3 high expression were resistant to taxane but sensitive to 5-fluoropyrimidines.
Conclusion
AGR3 might be a potential prognostic indicator in luminal B subtype of IDC patients of histological grade I-II. And patients with AGR3 high expression should be treated with chemotherapy regimens consisting of 5-fluoropyrimidines but no taxane.
Keyword
Figure
Reference
-
References
1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013; 63:11–30.
Article2. Mendes TF, Kluskens LD, Rodrigues LR. Triple negative breast cancer: nanosolutions for a big challenge. Adv Sci (Weinh). 2015; 2:1500053.
Article3. Abdel-Hafiz HA. Epigenetic mechanisms of tamoxifen resistance in luminal breast cancer. Diseases. 2017; 5:E16.
Article4. Hart CD, Sanna G, Siclari O, Biganzoli L, Di Leo A. Defining optimal duration and predicting benefit from chemotherapy in patients with luminal-like subtypes. Breast. 2015; 24 Suppl 2:S136–42.
Article5. Tran B, Bedard PL. Luminal-B breast cancer and novel therapeutic targets. Breast Cancer Res. 2011; 13:221.
Article6. Ivanova AS, Tereshina MB, Ermakova GV, Belousov VV, Zaraisky AG. Agr genes, missing in amniotes, are involved in the body appendages regeneration in frog tadpoles. Sci Rep. 2013; 3:1279.
Article7. Thompson DA, Weigel RJ. hAG-2, the human homologue of the Xenopus laevis cement gland gene XAG-2, is coexpressed with estrogen receptor in breast cancer cell lines. Biochem Biophys Res Commun. 1998; 251:111–6.8. Fletcher GC, Patel S, Tyson K, Adam PJ, Schenker M, Loader JA, et al. hAG-2 and hAG-3, human homologues of genes involved in differentiation, are associated with oestrogen receptor-positive breast tumours and interact with metastasis gene C4.4a and dystroglycan. Br J Cancer. 2003; 88:579–85.
Article9. Wilson CL, Sims AH, Howell A, Miller CJ, Clarke RB. Effects of oestrogen on gene expression in epithelium and stroma of normal human breast tissue. Endocr Relat Cancer. 2006; 13:617–28.
Article10. Hrstka R, Nenutil R, Fourtouna A, Maslon MM, Naughton C, Langdon S, et al. The pro-metastatic protein anterior gradient-2 predicts poor prognosis in tamoxifen-treated breast cancers. Oncogene. 2010; 29:4838–47.
Article11. Park K, Chung YJ, So H, Kim K, Park J, Oh M, et al. AGR2, a mucinous ovarian cancer marker, promotes cell proliferation and migration. Exp Mol Med. 2011; 43:91–100.
Article12. Zhang JS, Gong A, Cheville JC, Smith DI, Young CY. AGR2, an androgen-inducible secretory protein overexpressed in prostate cancer. Genes Chromosomes Cancer. 2005; 43:249–59.
Article13. Pohler E, Craig AL, Cotton J, Lawrie L, Dillon JF, Ross P, et al. The Barrett’s antigen anterior gradient-2 silences the p53 transcriptional response to DNA damage. Mol Cell Proteomics. 2004; 3:534–47.
Article14. Fritzsche FR, Dahl E, Dankof A, Burkhardt M, Pahl S, Petersen I, et al. Expression of AGR2 in non small cell lung cancer. Histol Histopathol. 2007; 22:703–8.15. Ramachandran V, Arumugam T, Wang H, Logsdon CD. Anterior gradient 2 is expressed and secreted during the development of pancreatic cancer and promotes cancer cell survival. Cancer Res. 2008; 68:7811–8.
Article16. Adam PJ, Boyd R, Tyson KL, Fletcher GC, Stamps A, Hudson L, et al. Comprehensive proteomic analysis of breast cancer cell membranes reveals unique proteins with potential roles in clinical cancer. J Biol Chem. 2003; 278:6482–9.
Article17. Garczyk S, von Stillfried S, Antonopoulos W, Hartmann A, Schrauder MG, Fasching PA, et al. AGR3 in breast cancer: prognostic impact and suitable serum-based biomarker for early cancer detection. PLoS One. 2015; 10:e0122106.
Article18. Li H, Narahara H. 15-Deoxy-Δ(12,14)-prostaglandin J(2) induces growth inhibition, cell cycle arrest and apoptosis in human endometrial cancer cell lines. Int J Mol Med. 2013; 31:778–88.
Article19. Pascal LE, Vencio RZ, Page LS, Liebeskind ES, Shadle CP, Troisch P, et al. Gene expression relationship between prostate cancer cells of Gleason 3, 4 and normal epithelial cells as revealed by cell type-specific transcriptomes. BMC Cancer. 2009; 9:452.
Article20. Gray TA, MacLaine NJ, Michie CO, Bouchalova P, Murray E, Howie J, et al. Anterior Gradient-3: a novel biomarker for ovarian cancer that mediates cisplatin resistance in xenograft models. J Immunol Methods. 2012; 378:20–32.
Article21. Vaarala MH, Hirvikoski P, Kauppila S, Paavonen TK. Identification of androgen-regulated genes in human prostate. Mol Med Rep. 2012; 6:466–72.
Article22. Bu H, Schweiger MR, Manke T, Wunderlich A, Timmermann B, Kerick M, et al. Anterior gradient 2 and 3: two prototype androgen-responsive genes transcriptionally upregulated by androgens and by oestrogens in prostate cancer cells. FEBS J. 2013; 280:1249–66.23. Obacz J, Brychtova V, Podhorec J, Fabian P, Dobes P, Vojtesek B, et al. Anterior gradient protein 3 is associated with less aggressive tumors and better outcome of breast cancer patients. Onco Targets Ther. 2015; 8:1523–32.24. King ER, Tung CS, Tsang YT, Zu Z, Lok GT, Deavers MT, et al. The anterior gradient homolog 3 (AGR3) gene is associated with differentiation and survival in ovarian cancer. Am J Surg Pathol. 2011; 35:904–12.
Article25. Welboren WJ, van Driel MA, Janssen-Megens EM, van Heeringen SJ, Sweep FC, Span PN, et al. ChIP-Seq of ERalpha and RNA polymerase II defines genes differentially responding to ligands. EMBO J. 2009; 28:1418–28.26. Mhaidat NM, Thorne R, Zhang XD, Hersey P. Involvement of endoplasmic reticulum stress in Docetaxel-induced JNK-dependent apoptosis of human melanoma. Apoptosis. 2008; 13:1505–12.
Article27. McGrath EP, Logue SE, Mnich K, Deegan S, Jager R, Gorman AM, et al. The unfolded protein response in breast cancer. Cancers (Basel). 2018; 10:E344.
Article28. Ryu J, Park SG, Lee PY, Cho S, Lee DH, Kim GH, et al. Dimerization of pro-oncogenic protein Anterior Gradient 2 is required for the interaction with BiP/GRP78. Biochem Biophys Res Commun. 2013; 430:610–5.
Article29. Kim JK, Kang KA, Piao MJ, Ryu YS, Han X, Fernando PM, et al. Endoplasmic reticulum stress induces 5-fluorouracil resistance in human colon cancer cells. Environ Toxicol Pharmacol. 2016; 44:128–33.
Article30. Yun S, Han YS, Lee JH, Kim S, Lee SH. Enhanced susceptibility to 5-fluorouracil in human colon cancer cells by silencing of GRP78. Anticancer Res. 2017; 37:2975–84.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comment on “Histomorphological Factors Predicting the Response to Neoadjuvant Chemotherapy in Triple-Negative Breast Cancerâ€
- Correlation between Tumor Response to Neoadjuvant Chemotherapy and Patient Outcome in Breast Cancer
- Correlation between Tumor Response to Neoadjuvant Chemotherapy and Patient Outcome in Breast Cancer
- Treatment with Cisplatin and Etoposide Chemotherapy in Patient with Metastatic Breast Cancer
- Chemotherapy in Breast Cancer