Cancer Res Treat.
2020 Jan;52(1):189-206. 10.4143/crt.2019.122.
Diabetes Mellitus Is Associated with Inferior Prognosis in Patients with Chronic Lymphocytic Leukemia: A Propensity Score-Matched Analysis
- Affiliations
-
- 1Department of Endocrinology, The First Affiliated Hospital of Nanjing Medical University, Jiangsu Province Hospital, Nanjing, China
- 2Department of Hematology, The First Affiliated Hospital of Nanjing Medical University, Jiangsu Province Hospital, Collaborative Innovation Center for Cancer Personalized Medicine, Nanjing, China
- KMID: 2501213
- DOI: http://doi.org/10.4143/crt.2019.122
Abstract
- Purpose
Diabetes mellitus (DM) is associated with elevated cancer risk and poor survival outcome in malignancies. The objective of this study was to evaluate the prognostic value of preexisting DM in chronic lymphocytic leukemia (CLL).
Materials and Methods
Six hundred and thirty-three subjects with newly-diagnosed CLL between 2007 and 2016 were recruited. Propensity score-matched method was performed to balance baseline characteristics and eliminate possible bias. Univariate and multivariate Cox regression analyses screened the independent risk indicators for time-to-first-treatment (TTFT) and cancer-specific survival (CSS) of CLL. Receiver operator characteristic curves and the corresponding areas under the curve assessed the predictive accuracy of CLL–International Prognostic Index (IPI) together with DM.
Results
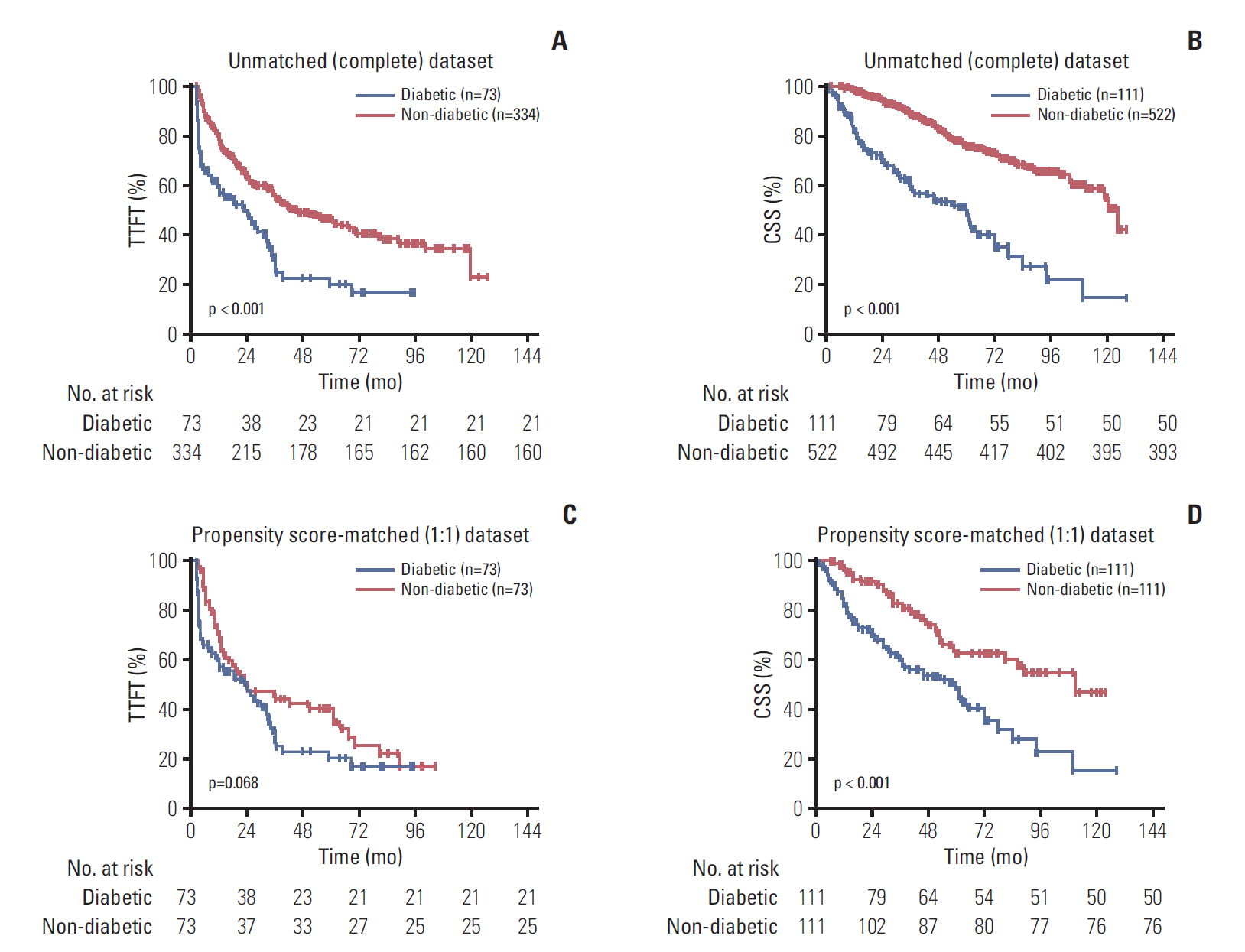
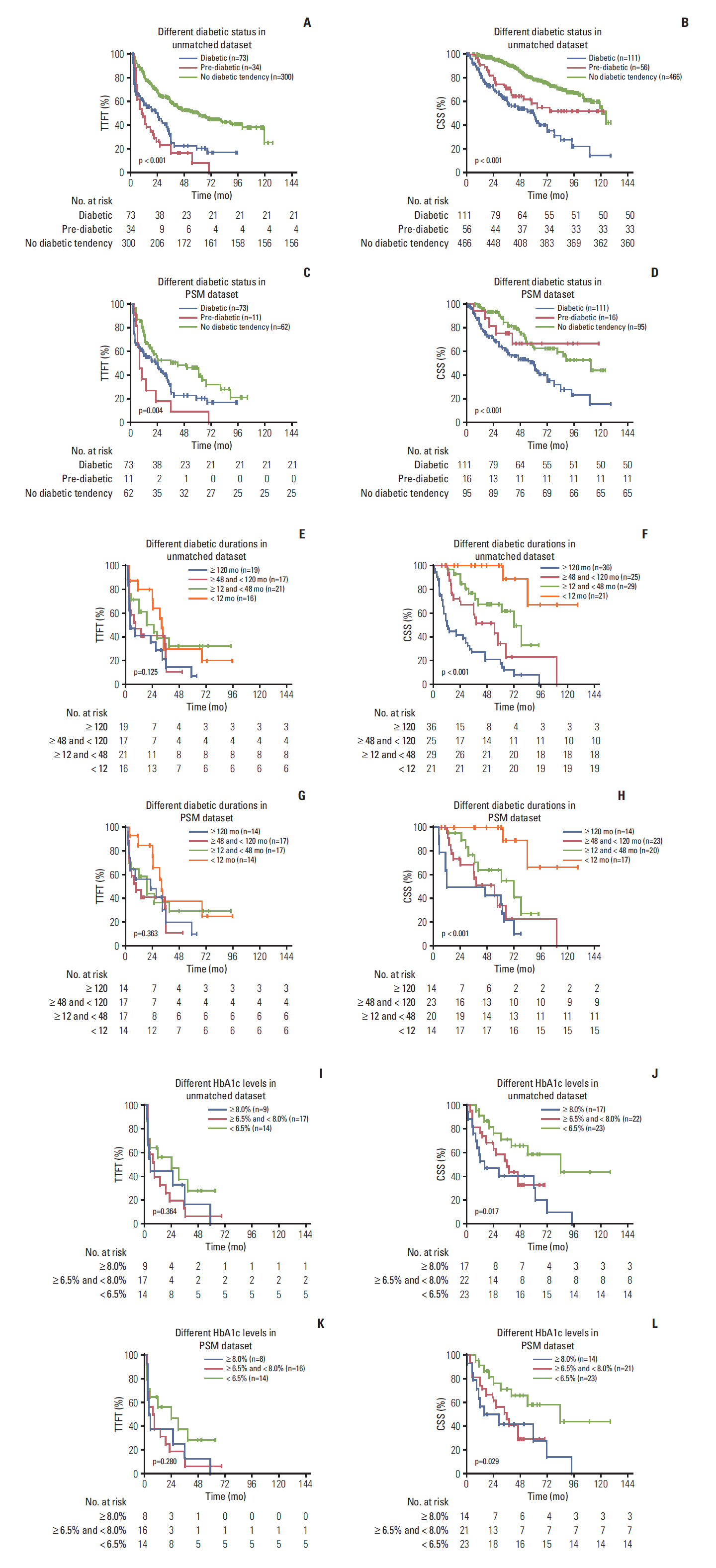
The results showed that 111 patients had pre-existing DM. In the propensity-matched cohort, DM was correlated with inferior TTFT and CSS in CLL patients, and it was an independent prognostic factor for both CSS and TTFT. Pre-diabetics also shared undesirable prognostic outcome compared with patients with no diabetic tendency, and a positive association between longer diabetic duration and poorer prognosis of CLL was identified. DM as one additional point to CLL-IPI had larger area under the curve compared with CLL-IPI alone in CSS prediction and could improve the prognostic capacity of CLL-IPI.
Conclusion
Pre-existing DM was found to be a valuable prognostic predictor and could help predict life expectancy and build refined prognostication models for CLL.
Figure
Reference
-
References
1. Mitri J, Castillo J, Pittas AG. Diabetes and risk of Non-Hodgkin’s lymphoma: a meta-analysis of observational studies. Diabetes Care. 2008; 31:2391–7.2. Castillo JJ, Mull N, Reagan JL, Nemr S, Mitri J. Increased incidence of non-Hodgkin lymphoma, leukemia, and myeloma in patients with diabetes mellitus type 2: a meta-analysis of observational studies. Blood. 2012; 119:4845–50.
Article3. Chao C, Page JH. Type 2 diabetes mellitus and risk of non-Hodgkin lymphoma: a systematic review and meta-analysis. Am J Epidemiol. 2008; 168:471–80.
Article4. Manda SO, Feltbower RG, Gilthorpe MS. Investigating spatiotemporal similarities in the epidemiology of childhood leukaemia and diabetes. Eur J Epidemiol. 2009; 24:743–52.
Article5. Malavasi F, Deaglio S, Damle R, Cutrona G, Ferrarini M, Chiorazzi N. CD38 and chronic lymphocytic leukemia: a decade later. Blood. 2011; 118:3470–8.
Article6. Hallek M, Fischer K, Fingerle-Rowson G, Fink AM, Busch R, Mayer J, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010; 376:1164–74.
Article7. Rassenti LZ, Huynh L, Toy TL, Chen L, Keating MJ, Gribben JG, et al. ZAP-70 compared with immunoglobulin heavy-chain gene mutation status as a predictor of disease progression in chronic lymphocytic leukemia. N Engl J Med. 2004; 351:893–901.
Article8. Bulian P, Shanafelt TD, Fegan C, Zucchetto A, Cro L, Nuckel H, et al. CD49d is the strongest flow cytometry-based predictor of overall survival in chronic lymphocytic leukemia. J Clin Oncol. 2014; 32:897–904.
Article9. Cui B, Chen L, Zhang S, Mraz M, Fecteau JF, Yu J, et al. MicroRNA-155 influences B-cell receptor signaling and associates with aggressive disease in chronic lymphocytic leukemia. Blood. 2014; 124:546–54.
Article10. Stilgenbauer S, Schnaiter A, Paschka P, Zenz T, Rossi M, Dohner K, et al. Gene mutations and treatment outcome in chronic lymphocytic leukemia: results from the CLL8 trial. Blood. 2014; 123:3247–54.
Article11. Mozessohn L, Earle C, Spaner D, Cheng SY, Kumar M, Buckstein R. The association of dyslipidemia with chronic lymphocytic leukemia: a population-based study. J Natl Cancer Inst. 2016; 109:djw226.
Article12. Xu W, Li JY, Pan JL, Qiu HR, Shen YF, Li L, et al. Interphase fluorescence in situ hybridization detection of cytogenetic abnormalities in B-cell chronic lymphocytic leukemia. Int J Hematol. 2007; 85:430–6.
Article13. Dong HJ, Zhou LT, Zhu DX, Wang DM, Fang C, Zhu HY, et al. The prognostic significance of TP53 mutations in Chinese patients with chronic lymphocytic leukemia is independent of del(17p13). Ann Hematol. 2011; 90:709–17.
Article14. Marinelli M, Ilari C, Xia Y, Del Giudice I, Cafforio L, Della Starza I, et al. Immunoglobulin gene rearrangements in Chinese and Italian patients with chronic lymphocytic leukemia. Oncotarget. 2016; 7:20520–31.
Article15. Xu W, Li JY, Wu YJ, Yu H, Shen QD, Li L, et al. Prognostic significance of ATM and TP53 deletions in Chinese patients with chronic lymphocytic leukemia. Leuk Res. 2008; 32:1071–7.
Article16. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998; 15:539–53.
Article17. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988; 44:837–45.
Article18. Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and cancer. Endocr Relat Cancer. 2009; 16:1103–23.
Article19. Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J, et al. Prevalence of diabetes among men and women in China. N Engl J Med. 2010; 362:1090–101.
Article20. Tseng CH. Diabetes, insulin use, and non-Hodgkin lymphoma mortality in Taiwan. Metabolism. 2012; 61:1003–9.
Article21. Lin SY, Hsieh MS, Chen LS, Chiu YH, Yen AM, Chen TH. Diabetes mellitus associated with the occurrence and prognosis of non-Hodgkin’s lymphoma. Eur J Cancer Prev. 2007; 16:471–8.
Article22. Hjalgrim H, Frisch M, Ekbom A, Kyvik KO, Melbye M, Green A. Cancer and diabetes: a follow-up study of two population-based cohorts of diabetic patients. J Intern Med. 1997; 241:471–5.23. Cerhan JR, Wallace RB, Folsom AR, Potter JD, Sellers TA, Zheng W, et al. Medical history risk factors for non-Hodgkin’s lymphoma in older women. J Natl Cancer Inst. 1997; 89:314–8.
Article24. Swafford AD, Howson JM, Davison LJ, Wallace C, Smyth DJ, Schuilenburg H, et al. An allele of IKZF1 (Ikaros) conferring susceptibility to childhood acute lymphoblastic leukemia protects against type 1 diabetes. Diabetes. 2011; 60:1041–4.
Article25. Zheng Z, Venkatapathy S, Rao G, Harrington CA. Expression profiling of B cell chronic lymphocytic leukemia suggests deficient CD1-mediated immunity, polarized cytokine response, altered adhesion and increased intracellular protein transport and processing of leukemic cells. Leukemia. 2002; 16:2429–37.
Article26. Beyer M, Kochanek M, Darabi K, Popov A, Jensen M, Endl E, et al. Reduced frequencies and suppressive function of CD4+ CD25hi regulatory T cells in patients with chronic lymphocytic leukemia after therapy with fludarabine. Blood. 2005; 106:2018–25.27. Pietropaolo M, Barinas-Mitchell E, Pietropaolo SL, Kuller LH, Trucco M. Evidence of islet cell autoimmunity in elderly patients with type 2 diabetes. Diabetes. 2000; 49:32–8.
Article28. Stephens JW, Hurel SJ, Cooper JA, Acharya J, Miller GJ, Humphries SE. A common functional variant in the interleukin-6 gene is associated with increased body mass index in subjects with type 2 diabetes mellitus. Mol Genet Metab. 2004; 82:180–6.
Article29. Fayad L, Keating MJ, Reuben JM, O’Brien S, Lee BN, Lerner S, et al. Interleukin-6 and interleukin-10 levels in chronic lymphocytic leukemia: correlation with phenotypic characteristics and outcome. Blood. 2001; 97:256–63.
Article30. Singer MK, Assem M, Abdel Ghaffar AB, Morcos NY. Role of TNF-alpha as a survival prognostic marker in chronic lymphocytic leukemia patients. Egypt J Immunol. 2011; 18:51–60.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Leukemia Cutis Associated with B-cell Chronic Lymphocytic Leukemia
- A Case of Nocardiosis in Patient with Chronic Lymphocytic Leukemia
- Hypoglycemia and Dementia Risk in Older Patients with Type 2 Diabetes Mellitus: A Propensity-Score Matched Analysis of a Population-Based Cohort Study (Diabetes Metab J 2020;44:125-33)
- Hypoglycemia and Dementia Risk in Older Patients with Type 2 Diabetes Mellitus: A Propensity-Score Matched Analysis of a Population-Based Cohort Study (Diabetes Metab J 2020;44:125-33)
- A case of transient diabetes mellitus and diabetic ketoacidosis induced by L-asparaginase and prednisolone administration in a patient with relapsed acute lymphocytic leukemia