Cancer Res Treat.
2020 Jan;52(1):149-166. 10.4143/crt.2019.183.
Therapeutic Co-targeting of WEE1 and ATM Downregulates PD-L1 Expression in Pancreatic Cancer
- Affiliations
-
- 1Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea
- 2Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea
- KMID: 2501210
- DOI: http://doi.org/10.4143/crt.2019.183
Abstract
- Purpose
Pancreatic cancer (PC) is one of the most lethal cancers worldwide, but there are currently no effective treatments. The DNA damage response (DDR) is under investigation for the development of novel anti-cancer drugs. Since DNA repair pathway alterations have been found frequently in PC, the purpose of this study was to test the DDR-targeting strategy in PC using WEE1 and ATM inhibitors.
Materials and Methods
We performed in vitro experiments using a total of ten human PC cell lines to evaluate antitumor effect of AZD1775 (WEE1 inhibitor) alone or combination with AZD0156 (ATM inhibitor). We established Capan-1–mouse model for in vivo experiments to confirm our findings.
Results
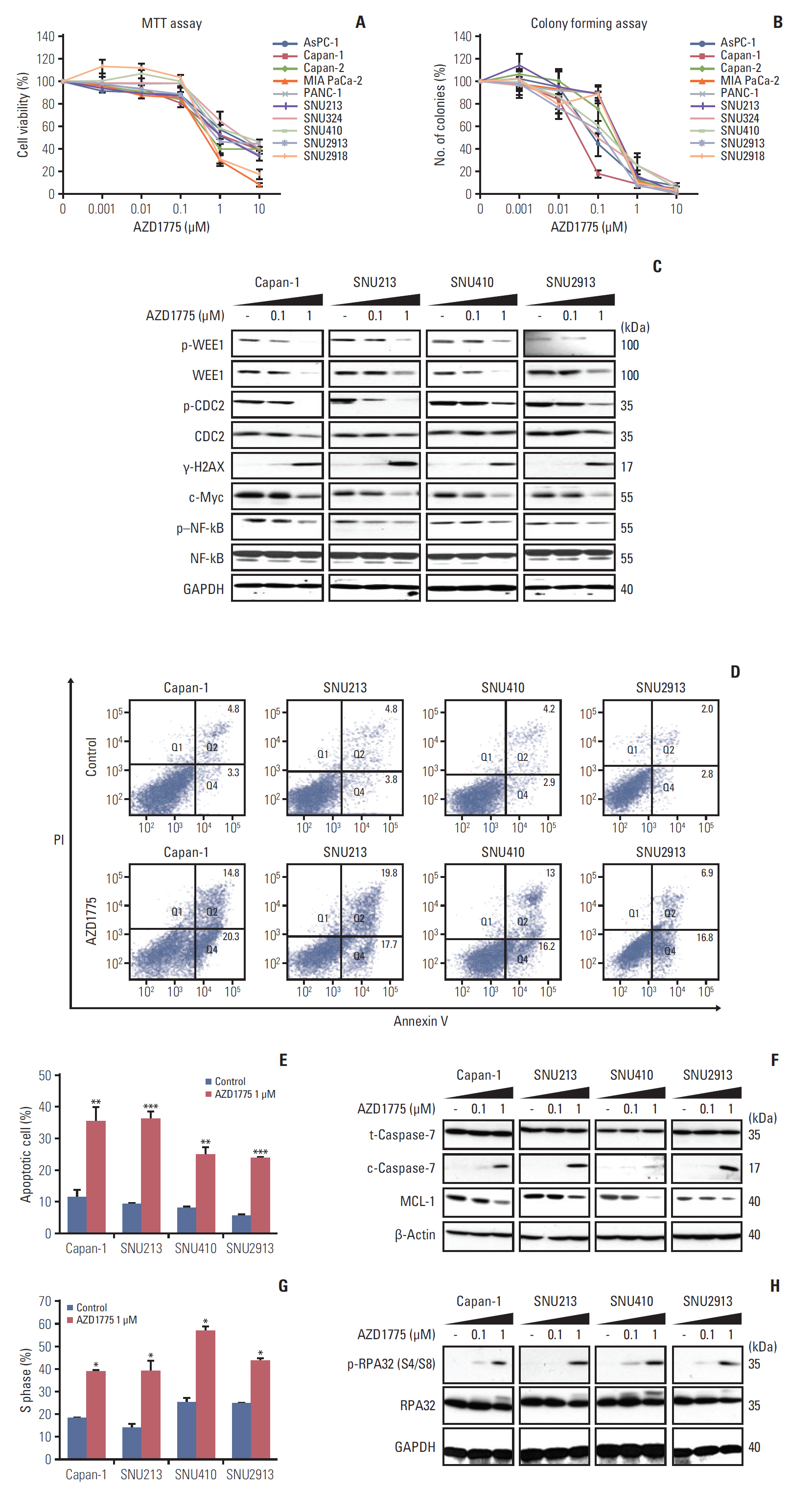
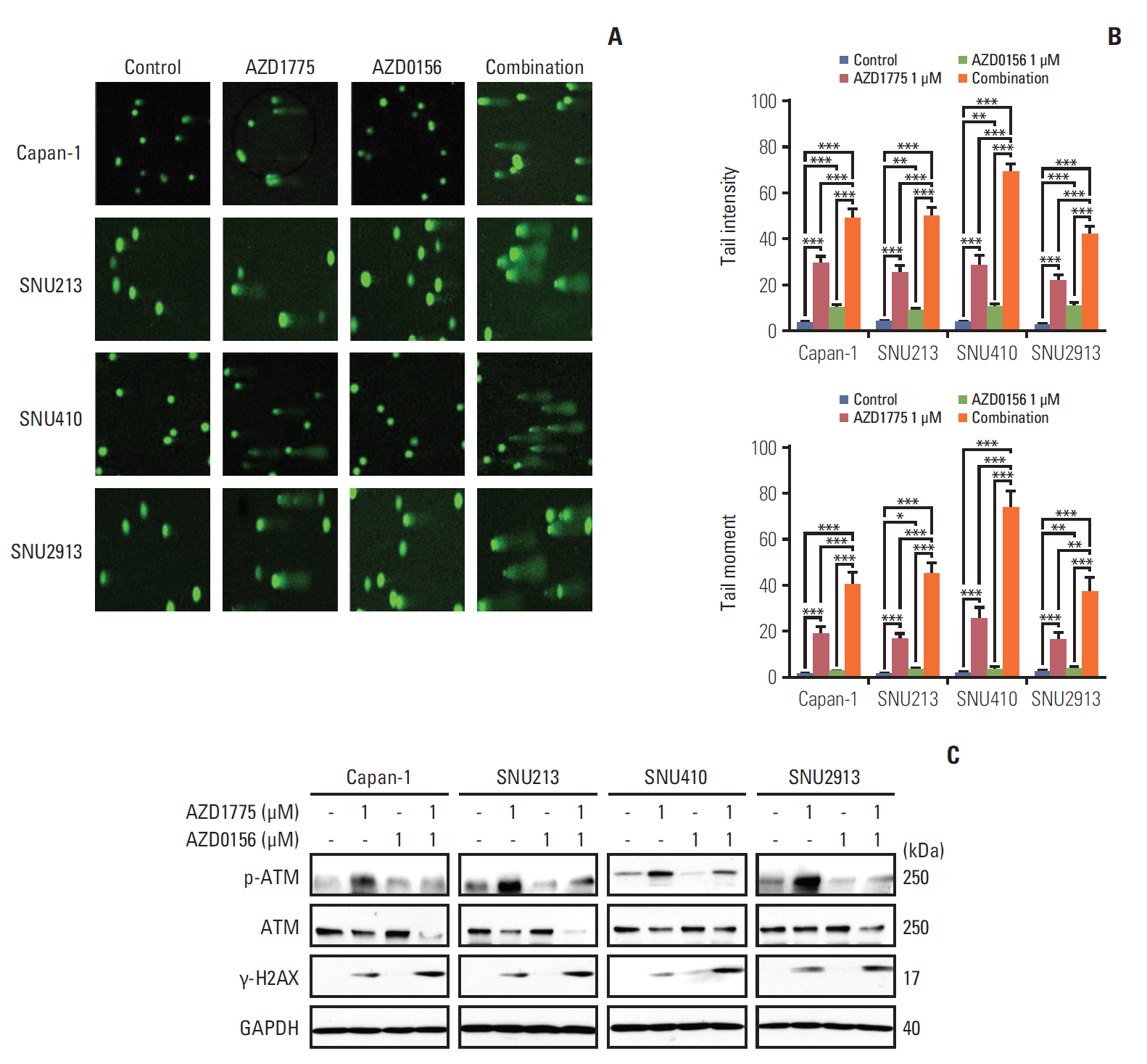
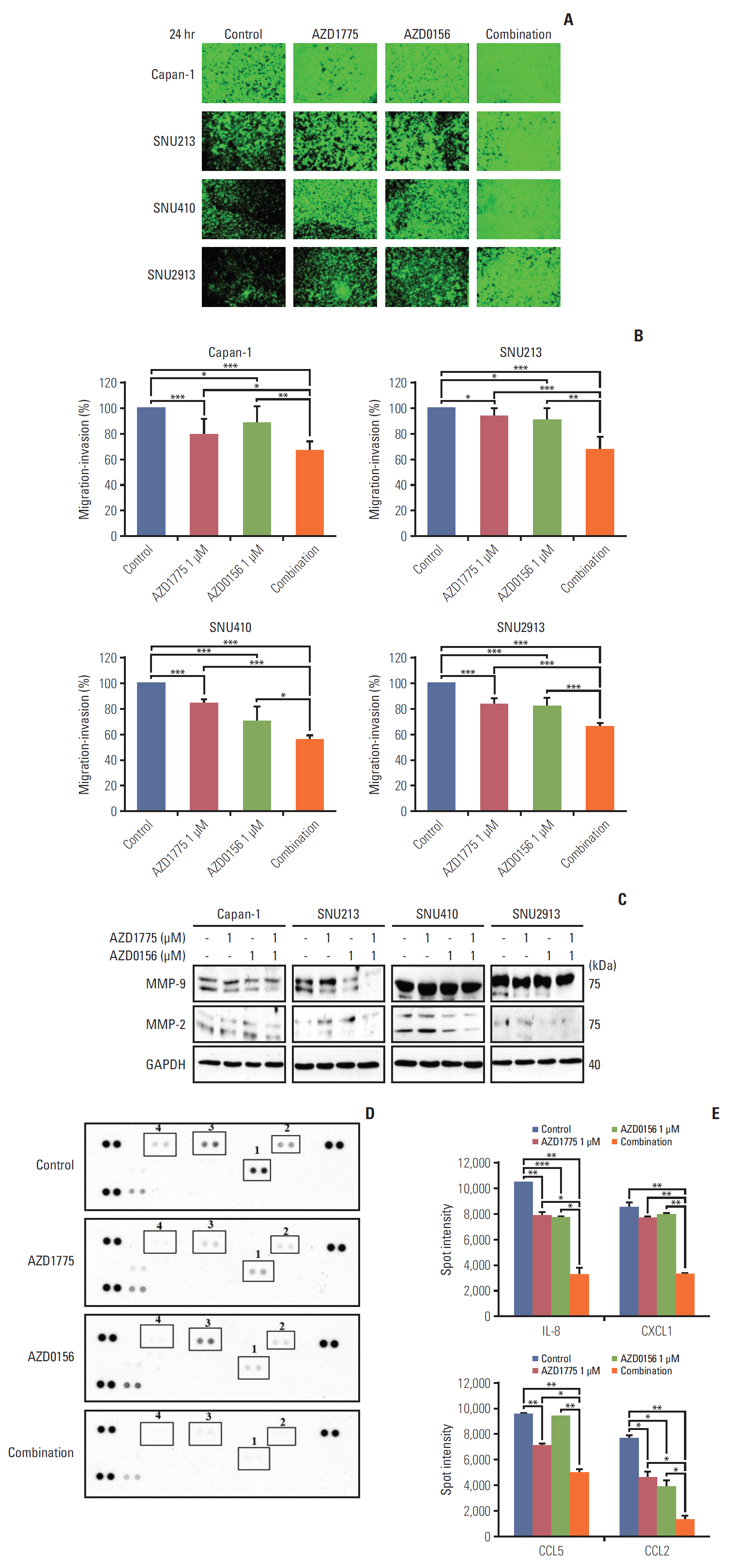
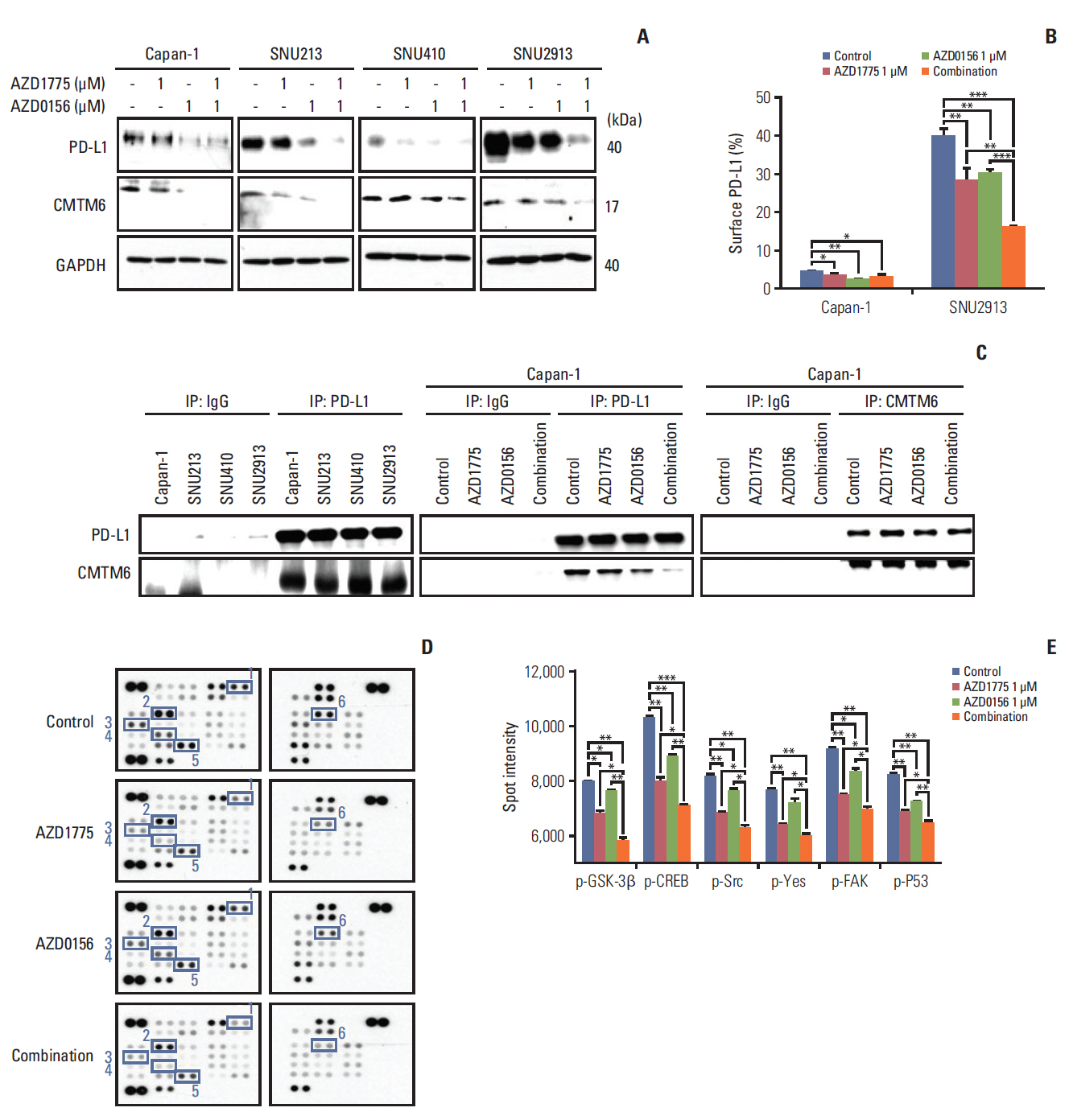
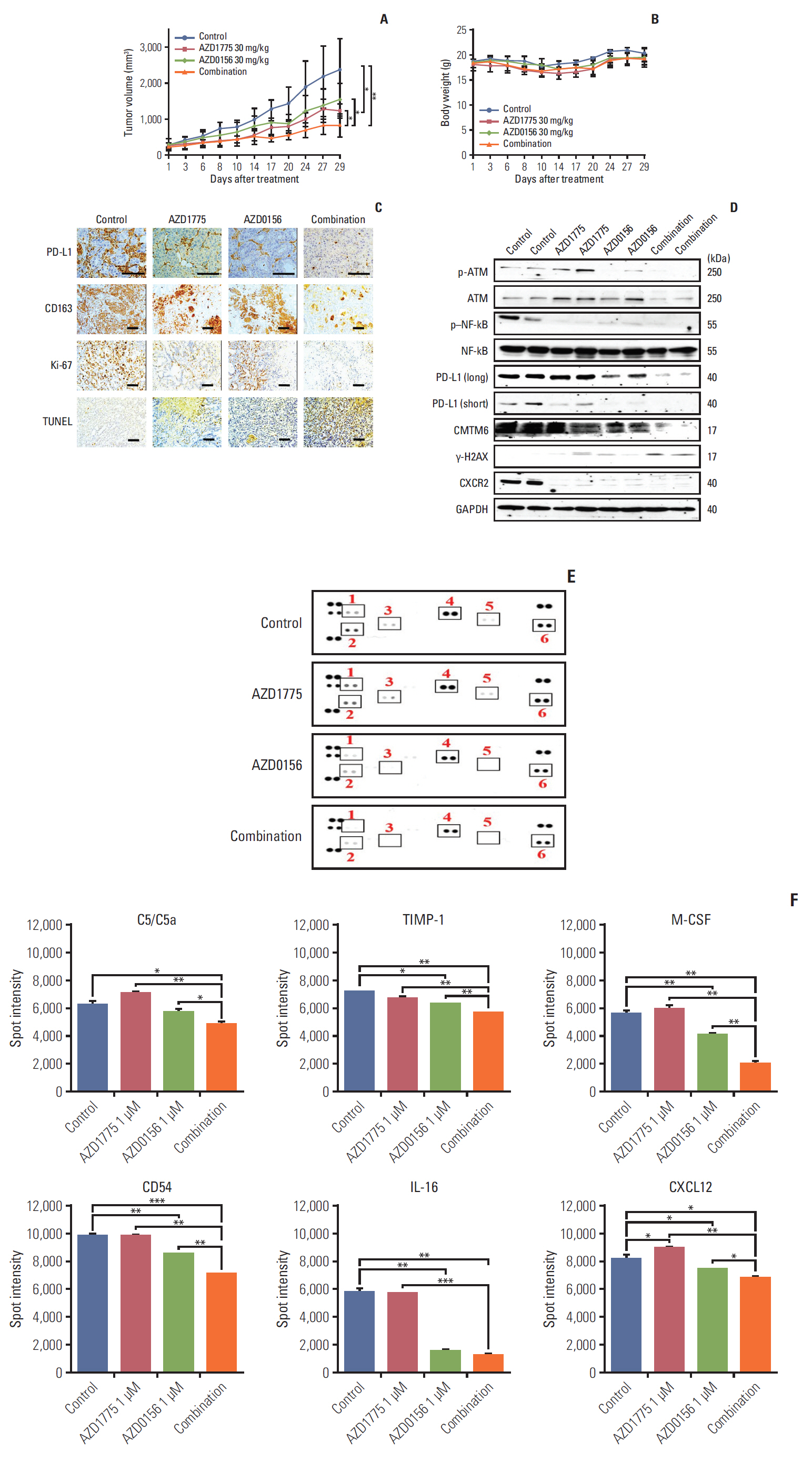
In our research, we found that WEE1 inhibitor (AZD1775) as single agent showed anti-tumor effects in PC cells, however, targeting WEE1 upregulated p-ATM level. Here, we observed that co-targeting of WEE1 and ATM acted synergistically to reduce cell proliferation and migration, and to induce DNA damage in vitro. Notably, inhibition of WEE1 or WEE1/ATM downregulated programmed cell death ligand 1 expression by blocking glycogen synthase kinase-3β serine 9 phosphorylation and decrease of CMTM6 expression. In Capan-1 mouse xenograft model, AZD1775 plus AZD0156 (ATM inhibitor) treatment reduced tumor growth and downregulated tumor expression of programmed cell death ligand 1, CMTM6, CD163, and CXCR2, all of which contribute to tumor immune evasion.
Conclusion
Dual blockade of WEE1 and ATM might be a potential therapeutic strategy for PC. Taken toget
Keyword
Figure
Reference
-
References
1. Bailey P, Chang DK, Nones K, Johns AL, Patch AM, Gingras MC, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016; 531:47–52.2. Choi Y, Oh DY, Kim K, Chie EK, Kim TY, Lee KH, et al. Concurrent chemoradiotherapy versus chemotherapy alone for unresectable locally advanced pancreatic cancer: a retrospective cohort study. Cancer Res Treat. 2016; 48:1045–55.
Article3. O’Connor MJ. Targeting the DNA damage response in cancer. Mol Cell. 2015; 60:547–60.
Article4. Richer AL, Cala JM, O’Brien K, Carson VM, Inge LJ, Whitsett TG. WEE1 kinase inhibitor AZD1775 has preclinical efficacy in LKB1-deficient non-small cell lung cancer. Cancer Res. 2017; 77:4663–72.
Article5. Kaufman B, Shapira-Frommer R, Schmutzler RK, Audeh MW, Friedlander M, Balmana J, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol. 2015; 33:244–50.
Article6. Chen CC, Kass EM, Yen WF, Ludwig T, Moynahan ME, Chaudhuri J, et al. ATM loss leads to synthetic lethality in BRCA1 BRCT mutant mice associated with exacerbated defects in homology-directed repair. Proc Natl Acad Sci U S A. 2017; 114:7665–70.
Article7. Mohni KN, Kavanaugh GM, Cortez D. ATR pathway inhibition is synthetically lethal in cancer cells with ERCC1 deficiency. Cancer Res. 2014; 74:2835–45.
Article8. Matheson CJ, Backos DS, Reigan P. Targeting WEE1 kinase in cancer. Trends Pharmacol Sci. 2016; 37:872–81.
Article9. Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018; 24:541–50.
Article10. Steele CW, Karim SA, Leach JD, Bailey P, Upstill-Goddard R, Rishi L, et al. CXCR2 inhibition profoundly suppresses metastases and augments immunotherapy in pancreatic ductal adenocarcinoma. Cancer Cell. 2016; 29:832–45.
Article11. Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013; 39:1–10.
Article12. Burr ML, Sparbier CE, Chan YC, Williamson JC, Woods K, Beavis PA, et al. CMTM6 maintains the expression of PD-L1 and regulates anti-tumour immunity. Nature. 2017; 549:101–5.
Article13. Chatzinikolaou G, Karakasilioti I, Garinis GA. DNA damage and innate immunity: links and trade-offs. Trends Immunol. 2014; 35:429–35.
Article14. Rosado MM, Bennici E, Novelli F, Pioli C. Beyond DNA repair, the immunological role of PARP-1 and its siblings. Immunology. 2013; 139:428–37.
Article15. Sato H, Niimi A, Yasuhara T, Permata TB, Hagiwara Y, Isono M, et al. DNA double-strand break repair pathway regulates PD-L1 expression in cancer cells. Nat Commun. 2017; 8:1751.
Article16. Jiao S, Xia W, Yamaguchi H, Wei Y, Chen MK, Hsu JM, et al. PARP inhibitor upregulates PD-L1 expression and enhances cancer-associated immunosuppression. Clin Cancer Res. 2017; 23:3711–20.
Article17. Farina AR, Mackay AR. Gelatinase B/MMP-9 in tumour pathogenesis and progression. Cancers (Basel). 2014; 6:240–96.
Article18. Gialeli C, Theocharis AD, Karamanos NK. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 2011; 278:16–27.
Article19. Zarogoulidis P, Katsikogianni F, Tsiouda T, Sakkas A, Katsikogiannis N, Zarogoulidis K. Interleukin-8 and interleukin-17 for cancer. Cancer Invest. 2014; 32:197–205.
Article20. Chuang JY, Yang WH, Chen HT, Huang CY, Tan TW, Lin YT, et al. CCL5/CCR5 axis promotes the motility of human oral cancer cells. J Cell Physiol. 2009; 220:418–26.
Article21. Tang CH, Tsai CC. CCL2 increases MMP-9 expression and cell motility in human chondrosarcoma cells via the Ras/Raf/MEK/ERK/NF-κB signaling pathway. Biochem Pharmacol. 2012; 83:335–44.
Article22. Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015; 27:450–61.
Article23. Li CW, Lim SO, Xia W, Lee HH, Chan LC, Kuo CW, et al. Glycosylation and stabilization of programmed death ligand-1 suppresses T-cell activity. Nat Commun. 2016; 7:12632.
Article24. Pilie PG, Tang C, Mills GB, Yap TA. State-of-the-art strategies for targeting the DNA damage response in cancer. Nat Rev Clin Oncol. 2019; 16:81–104.
Article25. Chen D, Lin X, Gao J, Shen L, Li Z, Dong B, et al. Wee1 inhibitor AZD1775 combined with cisplatin potentiates anticancer activity against gastric cancer by increasing DNA damage and cell apoptosis. Biomed Res Int. 2018; 2018:5813292.
Article26. Chen WT, Ebelt ND, Stracker TH, Xhemalce B, Van Den Berg CL, Miller KM. ATM regulation of IL-8 links oxidative stress to cancer cell migration and invasion. Elife. 2015; 4:e07270.
Article27. Dalton HJ, Armaiz-Pena GN, Gonzalez-Villasana V, LopezBerestein G, Bar-Eli M, Sood AK. Monocyte subpopulations in angiogenesis. Cancer Res. 2014; 74:1287–93.
Article28. Kolli-Bouhafs K, Sick E, Noulet F, Gies JP, De Mey J, Ronde P. FAK competes for Src to promote migration against invasion in melanoma cells. Cell Death Dis. 2014; 5:e1379.
Article29. Zhang ZX, Zhang WN, Sun YY, Li YH, Xu ZM, Fu WN. CREB promotes laryngeal cancer cell migration via MYCT1/NAT10 axis. Onco Targets Ther. 2018; 11:1323–31.
Article30. Garcia-Diaz A, Shin DS, Moreno BH, Saco J, Escuin-Ordinas H, Rodriguez GA, et al. Interferon receptor signaling pathways regulating PD-L1 and PD-L2 expression. Cell Rep. 2017; 19:1189–201.
Article31. Asgarova A, Asgarov K, Godet Y, Peixoto P, Nadaradjane A, Boyer-Guittaut M, et al. PD-L1 expression is regulated by both DNA methylation and NF-kB during EMT signaling in non-small cell lung carcinoma. Oncoimmunology. 2018; 7:e1423170.
Article32. Wang H, Yao H, Li C, Shi H, Lan J, Li Z, et al. HIP1R targets PD-L1 to lysosomal degradation to alter T cell-mediated cytotoxicity. Nat Chem Biol. 2019; 15:42–50.
Article33. Aras S, Zaidi MR. TAMeless traitors: macrophages in cancer progression and metastasis. Br J Cancer. 2017; 117:1583–91.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Expression of Programmed Death-Ligand 1 on Immune Cells Is Related to a Better Prognosis in Biliary Tract Cancer
- PD-L1 Expression and Combined Status of PD-L1/PD-1–Positive Tumor Infiltrating Mononuclear Cell Density Predict Prognosis in Glioblastoma Patients
- Prognostic Perspectives of STING and PD-L1 Expression and Correlation with the Prognosis of Epstein-Barr Virus-Associated Gastric Cancers
- Immunohistochemical expression of programmed death-ligand 1 and CD8 in glioblastomas
- Immunohistochemical Expression of PD-L1 in Cutaneous Squamous Cell Carcinoma and Its Association with Risk of Metastasis