J Korean Med Sci.
2020 Apr;35(15):e103. 10.3346/jkms.2020.35.e103.
The Emerging Crisis of Stakeholders in Implant-based Augmentation Mammaplasty in Korea
- Affiliations
-
- 1The W Clinic, Seoul, Korea
- 2Department of Surgery, Breast and Thyroid Cancer Center, Ewha Womans University School of Medicine, Seoul, Korea
- 3Department of Radiology, Gachon University Gil Medical Center, Incheon, Korea
- 4Department of Pathology, Jangwon Medical Foundation, Seoul, Korea
- 5Department of Surgery, Breast Cancer Center, Gachon University Gil Medical Center, Incheon, Korea
- KMID: 2500190
- DOI: http://doi.org/10.3346/jkms.2020.35.e103
Abstract
- Background
Korea is no longer safe from the risk of breast implant-associated anaplastic large cell lymphoma (BIA-ALCL); the first reported case was a Korean woman in her 40s who had a 7-year-history of receiving an implant-based augmentation mammaplasty using a textured implant. We conducted this study to discuss the emerging crisis of stakeholders in implant-based augmentation mammaplasty and to propose a multi-disciplinary approach to early detection of its complications.
Methods
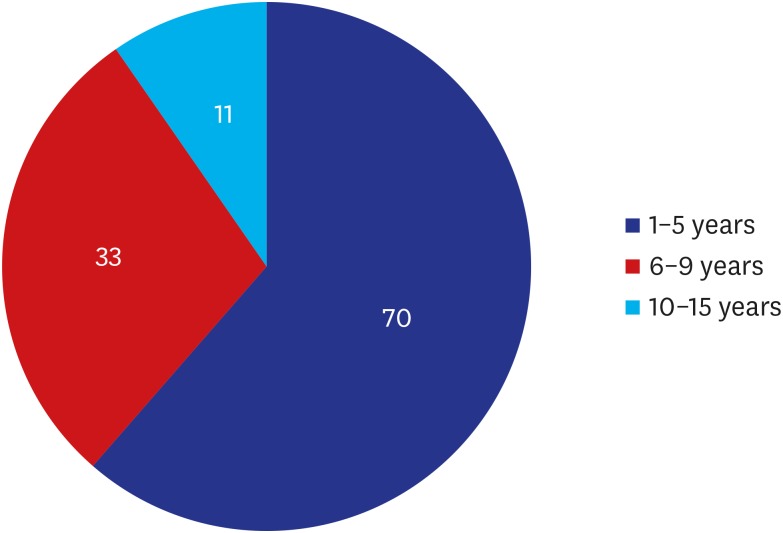
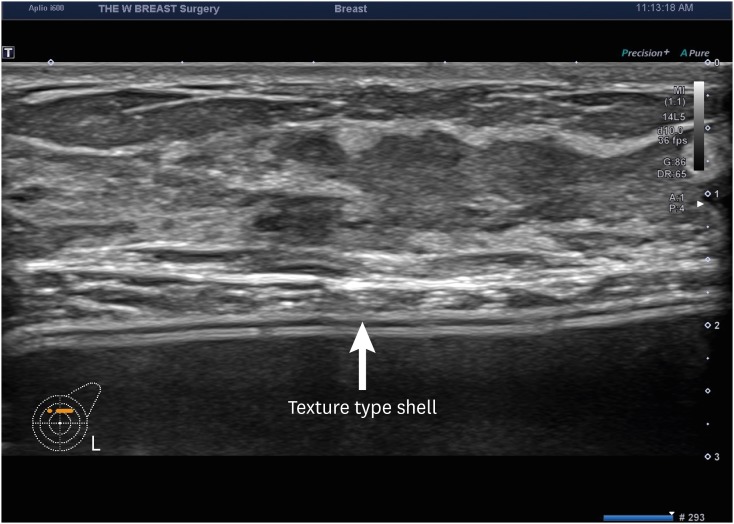
We analyzed medical examination data that was collected from patients who visited us between August 12 and September 27, 2019. We evaluated a total of 114 women (n = 114) in the current study. They were evaluated for whether they were in healthy condition. Moreover, their baseline characteristics were also examined; these included age, gender, height (cm), weight (kg), duration since surgery (years), possession of a breast implant card, the site of surgical incision, side of symptoms and reasons for outpatient visit. Furthermore, the patients were also evaluated for their subjective awareness of the manufacturer, surface and shape of the breast implant. Potential complications include malrotation, folding, seroma, capsule thickening, upside-down rotation, rupture, capsule mass and breast mass.
Results
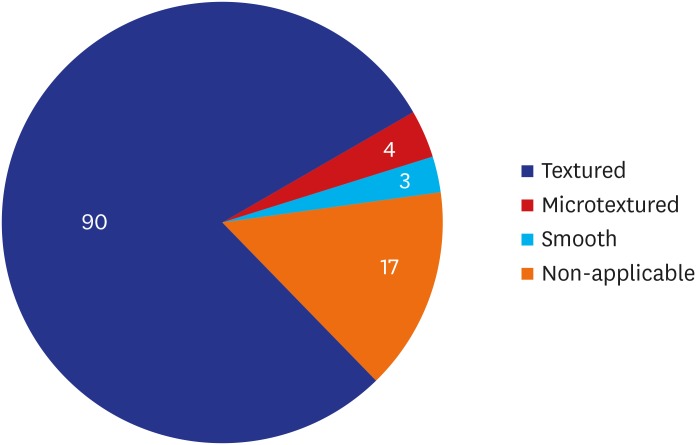
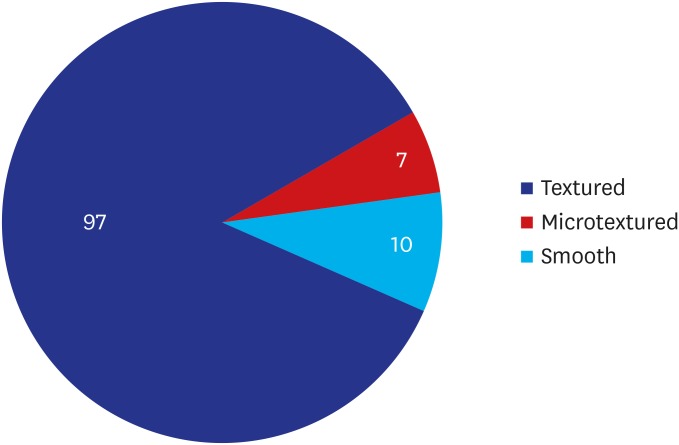
A majority of the patients had a past history of receiving textured implants. The corresponding percentage was 78.95% (90/114) and 85.09% (97/114) based on their subjective awareness of a breast implant and sonographic findings, respectively. That is, it was slightly increased with the use of a breast ultrasound.
Conclusion
Here, we propose the following approaches. First, patient data should be prospectively collected. By tracking outcomes and complications of an implant-based augmentation mammaplasty, both high-quality care and patient safety can be ensured. Second, stakeholders in implant-based augmentation mammaplasty should collaborate with customers and regulatory authorities. Third, surgeons should consider applying imaging modalities for early detection of postoperative complications.
Keyword
Figure
Reference
-
1. Ezekwudo DE, Ifabiyi T, Gbadamosi B, Haberichter K, Yu Z, Amin M, et al. Breast implant-associated anaplastic large cell lymphoma: a case report and review of the literature. Case Rep Oncol Med. 2017; 2017:6478467. PMID: 29225983.
Article2. Cordeiro PG, Ghione P, Ni A, Qunying H, Ganesan N, Galasso N, et al. Risk of breast implant associated anaplastic large cell lymphoma (BIA-ALCL) in a cohort of 3546 women prospectively followed long term after reconstruction with textured breast implants. J Plast Reconstr Aesthet Surg. 2020; DOI: 10.1016/j.bjps.2019.11.064.3. Cook JA, Sasor SE, Tholpady SS, Chu MW, Momeni A. Complexity of health news reporting on breast implant-associated anaplastic large cell lymphoma. Breast J. 2019; 25(1):163–165. PMID: 30592350.
Article4. U.S. Food and Drug Administration. General and plastic surgery devices panel of the medical devices advisory committee meeting announcement. Updated 2019. Accessed October 5, 2019. https://www.fda.gov/advisory-committees/advisory-committee-calendar/may-30-31-2019-general-and-plastic-surgery-devices-panel-medical-devices-advisory-committee-meeting.5. CISION PR Newswire. Allergan suspends sales and withdraws supply of textured breast implants in European markets. Updated 2018. Accessed October 5, 2019. https://www.prnewswire.com/news-releases/allergan-suspends-sales-and-withdraws-supply-of-textured-breast-implants-in-european-markets-300768847.html.6. Rohrich RJ. A review of breast-implant-associated anaplastic large cell lymphoma. Plast Reconstr Surg. 2019; 143(3 Suppl):1S–2S.7. Clemens MW. Current controversies in breast implant–associated anaplastic large cell lymphoma. Aesthet Surg J. 2019; 39(Suppl 1):S1–S2. PMID: 30715175.8. Deva AK. A perspective on the never-ending cycle of breast implant crises. Aesthet Surg J. 2019; 39(4):NP85–NP86. PMID: 30759184.
Article9. Swanson E. Textured breast implants, anaplastic large-cell lymphoma, and conflict of interest. Plast Reconstr Surg. 2017; 139(2):558e–559e.
Article10. Swanson E. Breast implant–associated anaplastic large cell lymphoma (BIA-ALCL): why the search for an infectious etiology may be irrelevant. Aesthet Surg J. 2017; 37(9):NP118–21. PMID: 29025238.
Article11. Swanson E, Mackay DR. Why the micromort concept falls short in breast implant–associated anaplastic large cell lymphoma (BIA-ALCL) risk analysis. Aesthet Surg J. 2018; 38(3):NP68–NP70. PMID: 29365043.
Article12. Swanson E, Brown T. A discussion of conflicts of interest in plastic surgery and possible remedies. Plast Reconstr Surg Glob Open. 2018; 6(12):e2043. PMID: 30656120.
Article13. de Jong D, Vasmel WL, de Boer JP, Verhave G, Barbé E, Casparie MK, et al. Anaplastic large-cell lymphoma in women with breast implants. JAMA. 2008; 300(17):2030–2035. PMID: 18984890.
Article14. Srinivasa DR, Miranda RN, Kaura A, Francis AM, Campanale A, Boldrini R, et al. Global adverse event reports of breast implant-associated ALCL: an international review of 40 government authority databases. Plast Reconstr Surg. 2017; 139(5):1029–1039. PMID: 28157770.15. ElHawary H, Ghazawi N, Efanov J, Izadpanah A. Abstract 31: a systematic review of breast-implant associated anaplastic large cell lymphoma (BIA-ALCL): past and current knowledge. Plast Reconstr Surg Glob Open. 2019; 7(4):Suppl. 22–23.16. Johnson L, O'Donoghue JM, McLean N, Turton P, Khan AA, Turner SD, et al. Breast implant associated anaplastic large cell lymphoma: The UK experience. Recommendations on its management and implications for informed consent. Eur J Surg Oncol. 2017; 43(8):1393–1401. PMID: 28596034.
Article17. Campanale A, Boldrini R. BIA-ALCL incidence: the variable to be included in the denominator. Plast Reconstr Surg. 2018; 141(5):779e.18. de Boer M, van Leeuwen FE, Hauptmann M, Overbeek LI, de Boer JP, Hijmering NJ, et al. Breast implants and the risk of anaplastic large-cell lymphoma in the breast. JAMA Oncol. 2018; 4(3):335–341. PMID: 29302687.
Article19. Fitzal F, Turner SD, Kenner L. Is breast implant-associated anaplastic large cell lymphoma a hazard of breast implant surgery? Open Biol. 2019; 9(4):190006. PMID: 30939983.
Article20. Kricheldorff J, Fallenberg EM, Solbach C, Gerber-Schäfer C, Rancsó C, Fritschen UV. Breast implant-associated lymphoma. Dtsch Arztebl Int. 2018; 115(38):628–635. PMID: 30373708.
Article21. Doren EL, Miranda RN, Selber JC, Garvey PB, Liu J, Medeiros LJ, et al. U.S. epidemiology of breast implant-associated anaplastic large cell lymphoma. Plast Reconstr Surg. 2017; 139(5):1042–1050. PMID: 28157769.
Article22. Lim J. Are ‘water drop’ breast implants a ticking time bomb in the chest? Updated 2019. Accessed September 10, 2019. http://www.koreaherald.com/view.php?ud=20190820000734.23. “In Korea between 2007 and 2018, women received a total of 222,470 textured breast implants with a risk of breast implant-associated analplastic large cell lymphoma”. Updated 2019. Accessed October 5, 2019. http://medigatenews.com/news/2630587104.24. Park AY, Seo BK, Cho KR, Woo OH. The utility of MicroPure™ ultrasound technique in assessing grouped microcalcifications without a mass on mammography. J Breast Cancer. 2016; 19(1):83–86. PMID: 27066098.
Article25. Sisa Journal. ‘Exceptional’ approval of a silicone gel-filled breast implant by the Korean Ministry of Food and Drug Safety. Updated 2017. Accessed October 6, 2019. https://www.sisajournal.com/news/articleView.html?idxno=170545.26. U.S. Food and Drug Administration. FDA issues warning letters to two breast implant manufacturers for failure to comply with post-approval study requirements. Updated 2019. Accessed September 25, 2019. https://www.fda.gov/news-events/press-announcements/fda-issues-warning-letters-two-breast-implant-manufacturers-failure-comply-post-approval-study.27. Han J, Jeong JH, Bang SI, Heo CY. BellaGel breast implant: 4-year results of a prospective cohort study. J Plast Surg Hand Surg. 2019; 53(4):232–239. PMID: 30888239.
Article28. Sung JY, Jeong JP, Moon DS, Kim MS, Kim HC, Choi WS, et al. Short-term safety of augmentation mammaplasty using the BellaGel implants in Korean women. Plast Reconstr Surg Glob Open. 2019; 7(12):e2566.
Article29. Sieber DA, Stark RY, Chase S, Schafer M, Adams WP Jr. Clinical evaluation of shaped gel breast implant rotation using high-resolution ultrasound. Aesthet Surg J. 2017; 37(3):290–296. PMID: 28207033.
Article30. Hall-Findlay EJ. Breast implant complication review: double capsules and late seromas. Plast Reconstr Surg. 2011; 127(1):56–66. PMID: 21200201.
Article31. Brody GS, Deapen D, Taylor CR, Pinter-Brown L, House-Lightner SR, Andersen JS, et al. Anaplastic large cell lymphoma occurring in women with breast implants: analysis of 173 cases. Plast Reconstr Surg. 2015; 135(3):695–705. PMID: 25490535.32. Hidalgo DA, Weinstein AL. Intraoperative comparison of anatomical versus round implants in breast augmentation: a randomized controlled trial. Plast Reconstr Surg. 2017; 139(3):587–596. PMID: 28234826.33. Friedman T, Davidovitch N, Scheflan M. Comparative double blind clinical study on round versus shaped cohesive gel implants. Aesthet Surg J. 2006; 26(5):530–536. PMID: 19338941.
Article34. Gahm J, Edsander-Nord A, Jurell G, Wickman M. No differences in aesthetic outcome or patient satisfaction between anatomically shaped and round expandable implants in bilateral breast reconstructions: a randomized study. Plast Reconstr Surg. 2010; 126(5):1419–1427. PMID: 20639801.
Article35. Hall-Findlay EJ. Discussion: late seromas and breast implants: theory and practice. Plast Reconstr Surg. 2012; 130(2):436–438. PMID: 22842415.36. Sforza M, Zaccheddu R, Alleruzzo A, Seno A, Mileto D, Paganelli A, et al. Preliminary 3-year evaluation of experience with SilkSurface and VelvetSurface motiva silicone breast implants: a single-center experience with 5813 consecutive breast augmentation cases. Aesthet Surg J. 2018; 38(suppl_2):S62–S73. PMID: 29040364.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Letter to the Editor: Discussion of the Article “The Emerging Crisis of Stakeholders in Implant-based Augmentation Mammaplasty in Korea”
- Breast Augmentation using Expandable Implants
- Augmentation Mammaplasty Using Cohesive Gel Implants
- Upside-Down Rotation of a Breast Implant with Double Capsule Formation after Aesthetic Breast Augmentation: A Case Report
- Augmentation Mammaplasty Using Implants: A Review