Blood Res.
2020 Mar;55(1):49-56. 10.5045/br.2020.55.1.49.
HIV-negative plasmablastic lymphoma: report of 8 cases and a comprehensive review of 394 published cases
- Affiliations
-
- 1Department of Lymphoma and Hematology, Hunan Cancer Hospital, Changsha, China. zhouhui@hnca.org.cn
- 2Department of Lymphoma and Hematology, The Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha, China.
- 3Department of Pathology, Fudan University Shanghai Cancer Center, Shanghai, China.
- 4Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China.
- 5Institute of Pathology, Fudan University, Shanghai, China.
- 6Department of Radiotherapy, Key Laboratory of Translational Radiation Oncology, Hunan Cancer Hospital, Changsha, China.
- 7Department of Oncology, Xiangya Hospital, Central South University, Changsha, China.
- 8Department of Oncology, Cancer Center of the Second Xiangya Hospital, Central South University, Changsha, China.
- KMID: 2472008
- DOI: http://doi.org/10.5045/br.2020.55.1.49
Abstract
- BACKGROUND
Human immunodeficiency virus (HIV)-negative plasmablastic lymphoma (PBL) is a rare entity of diffuse large B-cell lymphoma (DLBCL). The clinicopathological features of and optimal treatment for HIV-negative PBL remain largely unknown.
METHODS
To gain insight into this distinct lymphoma, we summarized the clinicopathologic characteristics of 8 unpublished HIV-negative PBLs and performed a comprehensive review of 394 published cases.
RESULTS
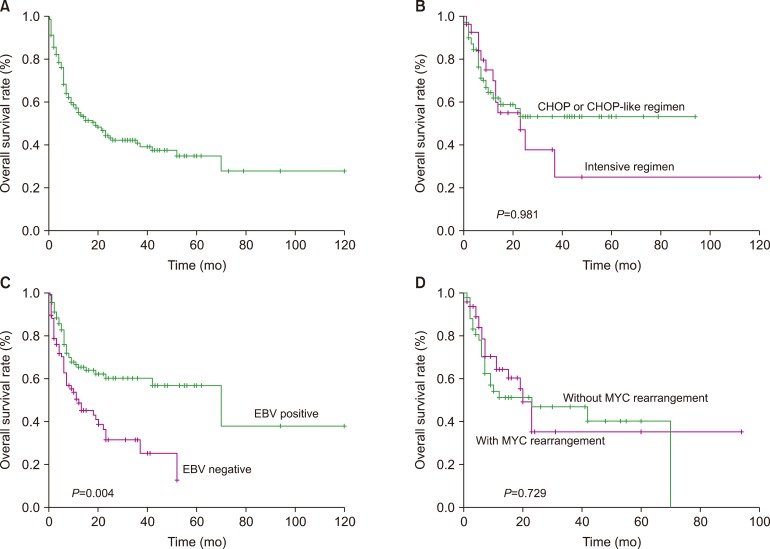
Of the 8 unpublished PBLs, the median patient age was 53.0 years. Four patients presented with stage IV disease. All 8 patients showed a plasma cell-like immunophenotype. Of the six patients who received anthracycline-based chemotherapy, including two who received bortezomib, three patients achieved a continuous complete response, two patients died due to disease progression, and one patient was lost to follow-up. The other two patients achieved continuous complete response after receiving chemotherapy combined with radiotherapy and surgery. Of the 402 patients, the majority were male, with a mean age of 58.0 years. EBV infection was detected in 55.7% of the patients. The median survival times of the patients who received CHOP or CHOP-like regimens and intensive regimens were not reached and 23.0 months, respectively, and the intensive regimen did not improve the survival outcome (P=0.981). Multivariate analysis showed that EBER remained the only independent factor affecting overall survival (OS).
CONCLUSION
HIV-negative PBL is a distinct entity with a predilection for elderly and immunosuppressed individuals. Intensive chemotherapy had no apparent survival benefits over the CHOP regimen in terms of OS; the prognosis of this disease is poor with current chemotherapy methods, and treatment remains a challenge.
MeSH Terms
Figure
Reference
-
1. Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011; 117:5019–5032. PMID: 21300984.
Article2. Gu X, Zheng R, Xia C, et al. Interactions between life expectancy and the incidence and mortality rates of cancer in China: a population-based cluster analysis. Cancer Commun (Lond). 2018; 38:44. PMID: 29970165.
Article3. Saba NS, Dang D, Saba J, et al. Bortezomib in plasmablastic lymphoma: a case report and review of the literature. Onkologie. 2013; 36:287–291. PMID: 23689224.
Article4. Cao C, Liu T, Zhu H, Wang L, Kai S, Xiang B. Bortezomib-contained chemotherapy and thalidomide combined with CHOP (Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone) play promising roles in plasmablastic lymphoma: a case report and literature review. Clin Lymphoma Myeloma Leuk. 2014; 14:e145–e150. PMID: 25225082.
Article5. Yan M, Dong Z, Zhao F, et al. CD20-positive plasmablastic lymphoma with excellent response to bortezomib combined with rituximab. Eur J Haematol. 2014; 93:77–80. PMID: 24528507.
Article6. Castillo JJ, Reagan JL, Sikov WM, Winer ES. Bortezomib in combination with infusional dose-adjusted EPOCH for the treatment of plasmablastic lymphoma. Br J Haematol. 2015; 169:352–355. PMID: 25612847.
Article7. Zanelli M, Ragazzi M, Valli R, et al. Unique presentation of a plasmablastic lymphoma superficially involving the entire large bowel. Pathol Res Pract. 2015; 211:1030–1033. PMID: 26459980.
Article8. Cencini E, Fabbri A, Guerrini S, Mazzei MA, Rossi V, Bocchia M. Long-term remission in a case of plasmablastic lymphoma treated with COMP (cyclophosphamide, liposomal doxorubicin, vincristine, prednisone) and bortezomib. Eur J Haematol. 2016; 96:650–654. PMID: 26715026.
Article9. Fedele PL, Gregory GP, Gilbertson M, et al. Infusional dose-adjusted epoch plus bortezomib for the treatment of plasmablastic lymphoma. Ann Hematol. 2016; 95:667–668. PMID: 26801792.
Article10. Konishi Y, Kaneko H, Nishi K, Imada K. Plasmablastic lymphoma of the gallbladder. Int J Hematol. 2016; 104:1–3. PMID: 27059870.
Article11. Li F, Ding W, Zuo Z, et al. Plasmablastic lymphoma: a clinico-pathologic analysis of 11 cases with review of literature. Zhonghua Bing Li Xue Za Zhi. 2016; 45:37–42. PMID: 26791552.12. Han X, Hu LX, Ouyang MQ, Duan MH, Zhou DB. Clinical characteristics and survival analysis of eight cases HIV-negative plasmablastic lymphoma. Zhonghua Xue Ye Xue Za Zhi. 2017; 38:290–294. PMID: 28468089.13. Lipstein M, O'Connor O, Montanari F, Paoluzzi L, Bongero D, Bhagat G. Bortezomib-induced tumor lysis syndrome in a patient with HIV-negative plasmablastic lymphoma. Clin Lymphoma Myeloma Leuk. 2010; 10:E43–E46. PMID: 21856550.
Article14. Castillo JJ, Reagan JL. Plasmablastic lymphoma: a systematic review. ScientificWorldJournal. 2011; 11:687–696. PMID: 21442146.
Article15. Castillo JJ, Furman M, Beltrán BE, et al. Human immunodeficiency virus-associated plasmablastic lymphoma: poor prognosis in the era of highly active antiretroviral therapy. Cancer. 2012; 118:5270–5277. PMID: 22510767.16. Laurent C, Fabiani B, Do C, et al. Immune-checkpoint expression in Epstein-Barr virus positive and negative plasmablastic lymphoma: a clinical and pathological study in 82 patients. Haematologica. 2016; 101:976–984. PMID: 27175027.
Article17. Matsuki E, Miyakawa Y, Asakawa S, et al. Identification of loss of p16 expression and upregulation of MDR-1 as genetic events resulting from two novel chromosomal translocations found in a plasmablastic lymphoma of the uterus. Clin Cancer Res. 2011; 17:2101–2109. PMID: 21325069.
Article18. Montes-Moreno S, Martinez-Magunacelaya N, Zecchini-Barrese T, et al. Plasmablastic lymphoma phenotype is determined by genetic alterations in MYC and PRDM1. Mod Pathol. 2017; 30:85–94.
Article19. Bogusz AM, Seegmiller AC, Garcia R, Shang P, Ashfaq R, Chen W. Plasmablastic lymphomas with MYC/IgH rearrangement: report of three cases and review of the literature. Am J Clin Pathol. 2009; 132:597–605. PMID: 19762538.20. Valera A, Balagué O, Colomo L, et al. IG/MYC rearrangements are the main cytogenetic alteration in plasmablastic lymphomas. Am J Surg Pathol. 2010; 34:1686–1694. PMID: 20962620.
Article21. Liu JJ, Zhang L, Ayala E, et al. Human immunodeficiency virus (HIV)-negative plasmablastic lymphoma: a single institutional experience and literature review. Leuk Res. 2011; 35:1571–1577. PMID: 21752466.
Article22. Liu M, Liu B, Liu B, et al. Human immunodeficiency virus-negative plasmablastic lymphoma: a comprehensive analysis of 114 cases. Oncol Rep. 2015; 33:1615–1620. PMID: 25695332.
Article23. Castillo J, Pantanowitz L, Dezube BJ. HIV-associated plasmablastic lymphoma: lessons learned from 112 published cases. Am J Hematol. 2008; 83:804–809. PMID: 18756521.
Article24. Morscio J, Dierickx D, Nijs J, et al. Clinicopathologic comparison of plasmablastic lymphoma in HIV-positive, immunocompetent, and posttransplant patients: single-center series of 25 cases and meta-analysis of 277 reported cases. Am J Surg Pathol. 2014; 38:875–886. PMID: 24832164.25. Lin L, Zhang X, Dong M, et al. Human immunodeficiency virus-negative plasmablastic lymphoma: A case report and literature review. Medicine (Baltimore). 2017; 96:e6171. PMID: 28207555.26. Castillo JJ, Winer ES, Stachurski D, et al. Clinical and pathological differences between human immunodeficiency virus-positive and human immunodeficiency virus-negative patients with plasmablastic lymphoma. Leuk Lymphoma. 2010; 51:2047–2053. PMID: 20919850.
Article27. Castillo JJ, Bibas M, Miranda RN. The biology and treatment of plasmablastic lymphoma. Blood. 2015; 125:2323–2330. PMID: 25636338.
Article28. He XH, Li B, Yang S, et al. R-CHOP regimen can significantly decrease the risk of disease relapse and progression in patients with non-germinal center B-cell subtype diffuse large B-cell lymphoma. Chin J Cancer. 2012; 31:306–314. PMID: 22640627.
Article29. Horwitz SM, Zelenetz AD, Gordon LI, et al. NCCN Guidelines insights: Non-Hodgkin's lymphomas, version 3.2016. J Natl Compr Canc Netw. 2016; 14:1067–1079. PMID: 27587620.30. Castillo JJ, Winer ES, Stachurski D, et al. Prognostic factors in chemotherapy-treated patients with HIV-associated plasmablastic lymphoma. Oncologist. 2010; 15:293–299. PMID: 20167839.
Article31. Ye H, Desai A, Gong T, et al. Spontaneous regression of mantle cell lymphoma: a report of four cases. Cancer Commun (Lond). 2018; 38:30. PMID: 29843782.
Article32. Dittus C, Grover N, Ellsworth S, Tan X, Park SI. Bortezomib in combination with dose-adjusted EPOCH (etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin) induces longterm survival in patients with plasmablastic lymphoma: a retrospective analysis. Leuk Lymphoma. 2018; 59:2121–2127. PMID: 29303024.
Article33. Schmit JM, DeLaune J, Norkin M, Grosbach A. A case of plasmablastic lymphoma achieving complete response and durable remission after lenalidomide-based therapy. Oncol Res Treat. 2017; 40:46–48. PMID: 28095384.
Article34. Holderness BM, Malhotra S, Levy NB, Danilov AV. Brentuximab vedotin demonstrates activity in a patient with plasmablastic lymphoma arising from a background of chronic lymphocytic leukemia. J Clin Oncol. 2013; 31:e197–e199. PMID: 23509308.
Article35. Bibas M, Grisetti S, Alba L, Picchi G, Del Nonno F, Antinori A. Patient with HIV-associated plasmablastic lymphoma responding to bortezomib alone and in combination with dexamethasone, gemcitabine, oxaliplatin, cytarabine, and pegfilgrastim chemotherapy and lenalidomide alone. J Clin Oncol. 2010; 28:e704–e708. PMID: 20823416.
Article36. Al-Malki MM, Castillo JJ, Sloan JM, Re A. Hematopoietic cell transplantation for plasmablastic lymphoma: a review. Biol Blood Marrow Transplant. 2014; 20:1877–1884. PMID: 24946718.
Article37. Nishi K, Mitani S, Hatanaka K, Imada K. Successful cord blood transplantation for an HIV-negative patient with refractory plasmablastic lymphoma. Ann Hematol. 2017; 96:1057–1058. PMID: 28293711.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Plasmablastic Lymphoma in a Human Immunodeficiency Virus-negative Patient: A Case Report and Review of the Literature
- A case of plasmablastic lymphoma in the nasal cavity in a human immunodeficiency virus-negative patient
- A Case Report with Plasmablastic Lymphoma of the Jejunum
- Fine Needle Aspiration Cytology of the Plasmablastic Lymphoma in Human Immunodeficiency Virus(HIV) Negative Patient: A Case Report
- Plasmablastic Lymphoma Mistaken for Perianal Abscess in Patient with AIDS