J Rheum Dis.
2020 Jan;27(1):61-67. 10.4078/jrd.2020.27.1.61.
MicroRNA-10b Plays a Role in Bone Formation by Suppressing Interleukin-22 in Ankylosing Spondylitis
- Affiliations
-
- 1Department of Rheumatology, Research Institute of Medical Sciences, Chonnam National University Medical School and Hospital, Gwangju, Korea. ktj1562@chonnam.ac.kr
- 2Department of Rheumatology, Hanyang University Hospital for Rheumatic Diseases, Seoul, Korea.
- KMID: 2471963
- DOI: http://doi.org/10.4078/jrd.2020.27.1.61
Abstract
OBJECTIVE
The microRNA (miR)-10b is the T helper (Th) 17 cell specific in patients with ankylosing spondylitis (AS). The interleukin (IL)-22, which is closely related to Th17 cells, has been implicated in the regulation of new bone formation in experimental models. Therefore, the aim of this study was to evaluate whether miR-10b affects bone formation via the IL-22 pathway in AS.
METHODS
Primary CD4+ T cells from AS were purified and transfected with miR-10b, anti-miR-10b, or scramble. Cell-surface markers and cytokine expression were analyzed by flow cytometry and enzyme-linked immunosorbent assay. Primary bone-derived cells (BdCs) from the facet joints of the spine were isolated, then osteogenic differentiation of primary BdCs was performed. We assessed alkaline phosphatase (ALP) activity and staining of BdCs at early time points. Alizarin red S staining of BdCs was performed at late time points.
RESULTS
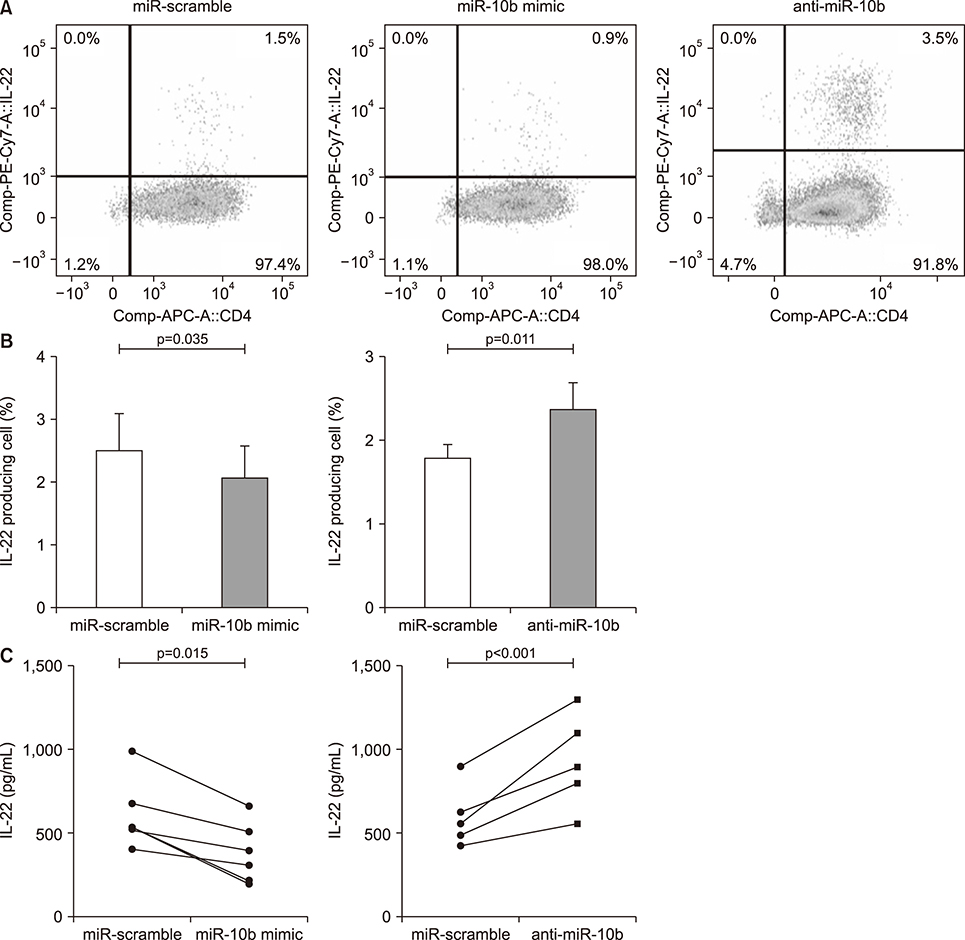
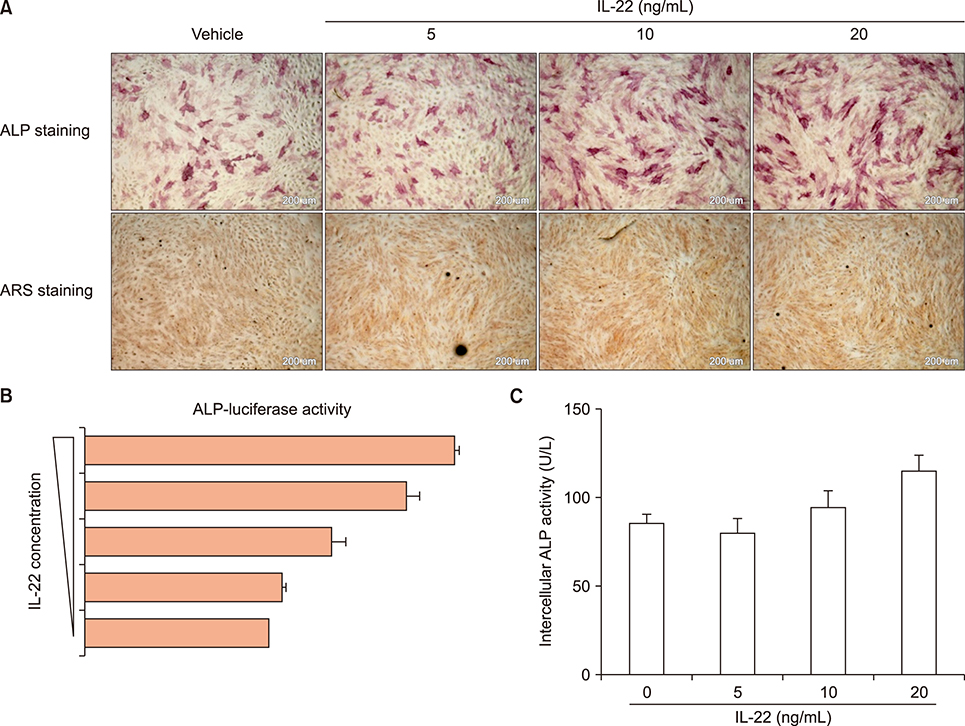
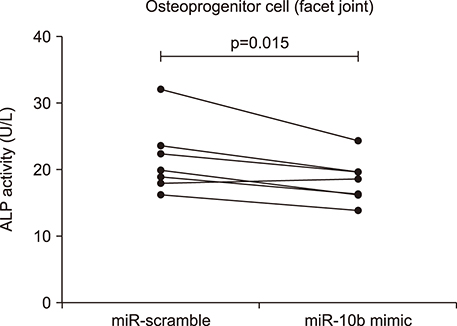
Overexpression of miR-10b reduced both IL-22 producing cell frequencies and cytokine production in T cells from the patients with AS. The IL-22 significantly increased ALP staining and bone mineralization. The ALP promotor activity of AS-BdCs was notably higher for the IL-22 concentration. The supernatants of the miR-10b overexpression group suppressed ALP activity on osteogenic progenitor cells from the facet joints of the spine in patients with AS.
CONCLUSION
Our data suggest that miR-10b suppresses IL-22 production, which was involved in osteogenic proliferation in AS. Therefore, miR-10b might be a potential therapeutic candidate for regulation of new bone formation in patients with AS.
Keyword
MeSH Terms
Figure
Cited by 3 articles
-
The Long, Dynamic Journey to the Elucidation of the Links Between Inflammation, Ectopic Bone Formation, and Wnt Signaling in Ankylosing Spondylitis
Seong-Ryul Kwon
J Rheum Dis. 2022;29(1):1-3. doi: 10.4078/jrd.2022.29.1.1.The Epidemiology and Treatment of Ankylosing Spondylitis in Korea
Seong-Ryul Kwon, Tae-Hwan Kim, Tae-Jong Kim, Won Park, Seung Cheol Shim
J Rheum Dis. 2022;29(4):193-199. doi: 10.4078/jrd.22.0023.Elevated BMPR2 expression amplifies osteoblast differentiation in ankylosing spondylitis
Sungsin Jo, Seung Hoon Lee, Chanhyeok Jeon, Hye-Ryeong Jo, Eunae Ko, Min Whangbo, Tae-Jong Kim, Ye-Soo Park, Tae-Hwan Kim
J Rheum Dis. 2023;30(4):243-250. doi: 10.4078/jrd.2023.0024.
Reference
-
1. Khan MA, van der. A wider spectrum of spondyloarthropathies. Semin Arthritis Rheum. 1990; 20:107–113.
Article2. Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007; 445:648–651.
Article3. Bowness P, Ridley A, Shaw J, Chan AT, Wong-Baeza I, Fleming M, et al. Th17 cells expressing KIR3DL2+ and responsive to HLA-B27 homodimers are increased in ankylosing spondylitis. J Immunol. 2011; 186:2672–2680.
Article4. Jandus C, Bioley G, Rivals JP, Dudler J, Speiser D, Romero P. Increased numbers of circulating polyfunctional Th17 memory cells in patients with seronegative spondylarthritides. Arthritis Rheum. 2008; 58:2307–2317.
Article5. Xueyi L, Lina C, Zhenbiao W, Qing H, Qiang L, Zhu P. Levels of circulating Th17 cells and regulatory T cells in ankylosing spondylitis patients with an inadequate response to anti-TNF-α therapy. J Clin Immunol. 2013; 33:151–161.
Article6. Bidad K, Salehi E, Jamshidi A, Saboor-Yaraghi AA, Oraei M, Meysamie A, et al. Effect of all-transretinoic acid on Th17 and T regulatory cell subsets in patients with ankylosing spondylitis. J Rheumatol. 2013; 40:476–483.
Article7. Shen H, Goodall JC, Hill Gaston JS. Frequency and phenotype of peripheral blood Th17 cells in ankylosing spondylitis and rheumatoid arthritis. Arthritis Rheum. 2009; 60:1647–1656.
Article8. Sherlock JP, Joyce-Shaikh B, Turner SP, Chao CC, Sathe M, Grein J, et al. IL-23 induces spondyloarthropathy by acting on ROR-γt+ CD3+CD4-CD8- entheseal resident T cells. Nat Med. 2012; 18:1069–1076.
Article9. El-Zayadi AA, Jones EA, Churchman SM, Baboolal TG, Cuthbert RJ, El-Jawhari JJ, et al. Interleukin-22 drives the proliferation, migration and osteogenic differentiation of mesenchymal stem cells: a novel cytokine that could contribute to new bone formation in spondyloarthropathies. Rheumatology (Oxford). 2017; 56:488–493.
Article10. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004; 116:281–297.11. O'Connell RM, Rao DS, Chaudhuri AA, Baltimore D. Physiological and pathological roles for microRNAs in the immune system. Nat Rev Immunol. 2010; 10:111–122.12. Pillai RS, Bhattacharyya SN, Filipowicz W. Repression of protein synthesis by miRNAs: how many mechanisms? Trends Cell Biol. 2007; 17:118–126.
Article13. Xiao C, Srinivasan L, Calado DP, Patterson HC, Zhang B, Wang J, et al. Lymphoproliferative disease and autoimmunity in mice with increased miR-17-92 expression in lymphocytes. Nat Immunol. 2008; 9:405–414.
Article14. Jin HM, Kim TJ, Choi JH, Kim MJ, Cho YN, Nam KI, et al. MicroRNA-155 as a proinflammatory regulator via SHIP-1 down-regulation in acute gouty arthritis. Arthritis Res Ther. 2014; 16:R88.
Article15. Thai TH, Calado DP, Casola S, Ansel KM, Xiao C, Xue Y, et al. Regulation of the germinal center response by microRNA-155. Science. 2007; 316:604–608.
Article16. Chen L, Al-Mossawi MH, Ridley A, Sekine T, Hammitzsch A, de Wit J, et al. miR-10b-5p is a novel Th17 regulator present in Th17 cells from ankylosing spondylitis. Ann Rheum Dis. 2017; 76:620–625.
Article17. Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006; 203:2271–2279.
Article18. Jo S, Koo BS, Lee B, Kwon E, Lee YL, Chung H, et al. A novel role for bone-derived cells in ankylosing spondylitis: focus on IL-23. Biochem Biophys Res Commun. 2017; 491:787–793.
Article19. Jo S, Kang S, Han J, Choi SH, Park YS, Sung IH, et al. Accelerated osteogenic differentiation of human bone-derived cells in ankylosing spondylitis. J Bone Miner Metab. 2018; 36:307–313.
Article20. Choi YH, Kim YJ, Jeong HM, Jin YH, Yeo CY, Lee KY. Akt enhances Runx2 protein stability by regulating Smurf2 function during osteoblast differentiation. FEBS J. 2014; 281:3656–3666.
Article21. Jo S, Wang SE, Lee YL, Kang S, Lee B, Han J, et al. IL-17A induces osteoblast differentiation by activating JAK2/STAT3 in ankylosing spondylitis. Arthritis Res Ther. 2018; 20:115.
Article22. Benjamin M, Toumi H, Suzuki D, Hayashi K, McGonagle D. Evidence for a distinctive pattern of bone formation in enthesophytes. Ann Rheum Dis. 2009; 68:1003–1010.
Article23. Lories RJ, Derese I, Luyten FP. Modulation of bone morphogenetic protein signaling inhibits the onset and progression of ankylosing enthesitis. J Clin Invest. 2005; 115:1571–1579.
Article24. Feng D, Kong X, Weng H, Park O, Wang H, Dooley S, et al. Interleukin-22 promotes proliferation of liver stem/progenitor cells in mice and patients with chronic hepatitis B virus infection. Gastroenterology. 2012; 143:188–198.
Article25. Baraliakos X, Haibel H, Listing J, Sieper J, Braun J. Continuous long-term anti-TNF therapy does not lead to an increase in the rate of new bone formation over 8 years in patients with ankylosing spondylitis. Ann Rheum Dis. 2014; 73:710–715.26. Poddubnyy D, Rudwaleit M, Haibel H, Listing J, Märker-Hermann E, Zeidler H, et al. Effect of non-steroidal anti-inflammatory drugs on radiographic spinal progression in patients with axial spondyloarthritis: results from the German Spondyloarthritis Inception Cohort. Ann Rheum Dis. 2012; 71:1616–1622.
Article27. Ma L, Teruya-Feldstein J, Weinberg RA. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007; 449:682–688.
Article28. Li Z, Lei H, Luo M, Wang Y, Dong L, Ma Y, et al. DNA methylation downregulated mir-10b acts as a tumor suppressor in gastric cancer. Gastric Cancer. 2015; 18:43–54.
Article29. Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen ZJ. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001; 412:346–351.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Interleukin-32 Gamma as a New Face in Inflammatory Bone Diseases
- A Case of Rapid Progressive Neurosyphilis in Patient with Ankylosing Spondylitis Who Is Treating Anti-interleukin 17A Monoclonal Antibody, Secukinumab
- Ankylosing Spondylitis: Prevention And Surgical Correction Of Deformity
- Clinieal Values of Single Photon Emission Computed Tomography ( SPECT ) in Ankylosing Spondylitis
- Tailored Treatment of Ankylosing Spondylitis