Nutr Res Pract.
2020 Apr;14(2):127-133. 10.4162/nrp.2020.14.2.127.
Luteolin attenuates migration and invasion of lung cancer cells via suppressing focal adhesion kinase and non-receptor tyrosine kinase signaling pathway
- Affiliations
-
- 1Department of Pharmacology, Faculty of Science, Prince of Songkla University, Songkhla, 90110, Thailand. wanida.su@psu.ac.th
- 2Department of Anatomy, Faculty of Science, Prince of Songkla University, Songkhla, 90110, Thailand.
- 3Department of Physiology, Faculty of Science, Prince of Songkla University, Songkhla, 90110, Thailand.
- KMID: 2471880
- DOI: http://doi.org/10.4162/nrp.2020.14.2.127
Abstract
- BACKGROUND/OBJECTIVES
Non-small cell lung cancer is mostly recognized among other types of lung cancer with a poor prognosis by cause of chemotherapeutic resistance and increased metastasis. Luteolin has been found to decrease cell metastasis. However, its underlying mechanisms remain unresolved. The objective of this study was to examine the effect (and its mechanism) of luteolin on the migration and invasion of human non-small cell lung cancer A549 cells.
MATERIALS/METHODS
Cell viability was investigated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. Wound healing and transwell assays were evaluated to assess migration and invasion, respectively. Western blot analysis and immunofluorescence were further performed to investigate the role of luteolin and its mechanisms of action.
RESULTS
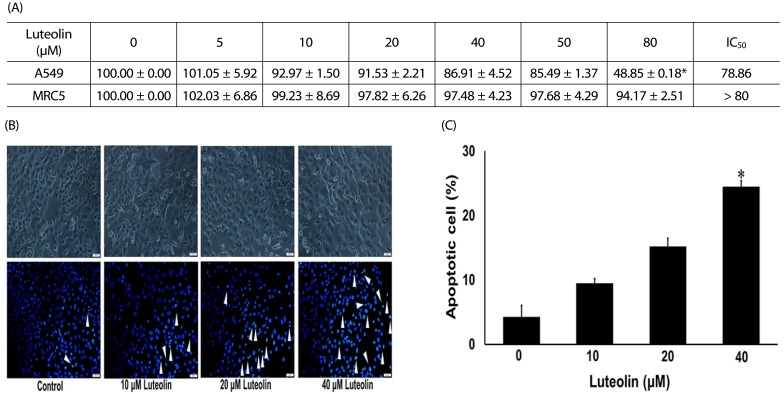
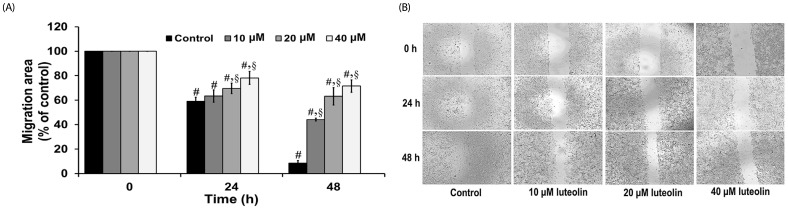
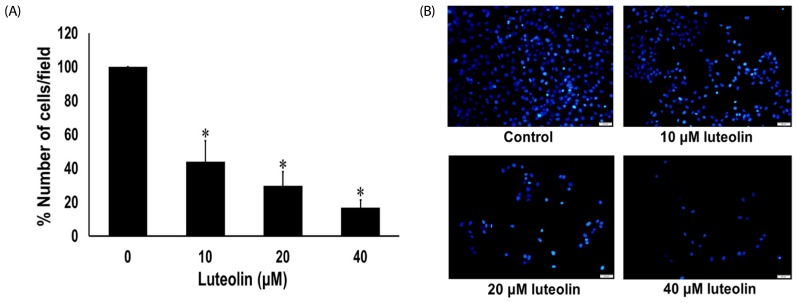
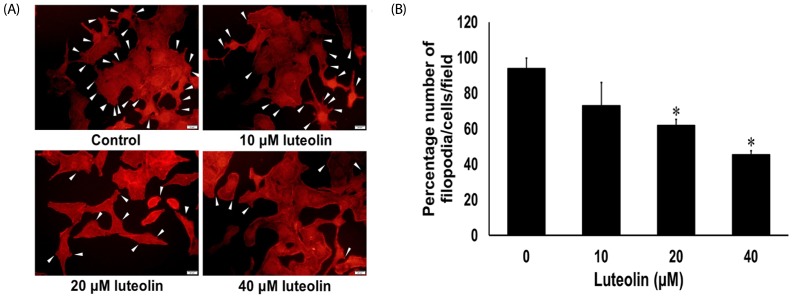
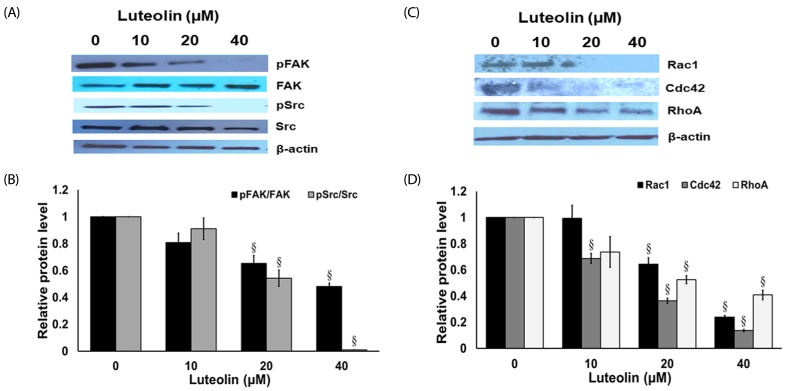
Administration with up to 40 µM luteolin showed no cytotoxic activity on lung cancer A549 cells or non-cancer MRC-5 cells. Additionally, luteolin at 20-40 µM significantly suppressed A549 cells' migration, invasion, and the formation of filopodia in a concentration-dependent manner at 24 h. This is similar with western blot analysis, which revealed diminished the phosphorylated focal adhesion kinase (pFAK), phosphorylated non-receptor tyrosine kinase (pSrc), Ras-related C3 botulinum toxin substrate 1 (Rac1), cell division control protein 42 (Cdc42), and Ras homolog gene family member A (RhoA) expression levels.
CONCLUSIONS
Overall, our data indicate that luteolin plays a role in controlling lung cancer cells' migration and invasion via Src/FAK and its downstream Rac1, Cdc42, and RhoA pathways. Luteolin might be considered a promising candidate for suppressing invasion and metastasis of lung cancer cells.
Keyword
MeSH Terms
-
Adenocarcinoma
Blotting, Western
Carcinoma, Non-Small-Cell Lung
Cell Division
Cell Survival
Flavonoids
Fluorescent Antibody Technique
Focal Adhesion Protein-Tyrosine Kinases*
Focal Adhesions*
Humans
Lung Neoplasms*
Lung*
Luteolin*
Neoplasm Metastasis
Prognosis
Protein-Tyrosine Kinases*
Pseudopodia
rac1 GTP-Binding Protein
Tyrosine*
Wound Healing
Flavonoids
Focal Adhesion Protein-Tyrosine Kinases
Luteolin
Protein-Tyrosine Kinases
Tyrosine
rac1 GTP-Binding Protein
Figure
Reference
-
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424. PMID: 30207593.
Article2. Sher T, Dy GK, Adjei AA. Small cell lung cancer. Mayo Clin Proc. 2008; 83:355–367. PMID: 18316005.
Article3. Artal Cortés Á, Calera Urquizu L, Hernando Cubero J. Adjuvant chemotherapy in non-small cell lung cancer: state-of-the-art. Transl Lung Cancer Res. 2015; 4:191–197. PMID: 25870801.4. Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998; 279:509–514. PMID: 9438836.
Article5. Ridley AJ. Rho GTPases and cell migration. J Cell Sci. 2001; 114:2713–2722. PMID: 11683406.
Article6. Iden S, Collard JG. Crosstalk between small GTPases and polarity proteins in cell polarization. Nat Rev Mol Cell Biol. 2008; 9:846–859. PMID: 18946474.
Article7. Tomar A, Schlaepfer DD. Focal adhesion kinase: switching between GAPs and GEFs in the regulation of cell motility. Curr Opin Cell Biol. 2009; 21:676–683. PMID: 19525103.
Article8. Lee BY, Timpson P, Horvath LG, Daly RJ. FAK signaling in human cancer as a target for therapeutics. Pharmacol Ther. 2015; 146:132–149. PMID: 25316657.
Article10. Zhang S, Yu D. Targeting Src family kinases in anti-cancer therapies: turning promise into triumph. Trends Pharmacol Sci. 2012; 33:122–128. PMID: 22153719.
Article11. Park GB, Kim D. Cigarette smoke-induced EGFR activation promotes epithelial mesenchymal migration of human retinal pigment epithelial cells through regulation of the FAK-mediated Syk/Src pathway. Mol Med Rep. 2018; 17:3563–3574. PMID: 29286114.
Article12. Mitra SK, Schlaepfer DD. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol. 2006; 18:516–523. PMID: 16919435.
Article13. Prateep A, Sumkhemthong S, Karnsomwan W, De-Eknamkul W, Chamni S, Chanvorachote P, Chaotham C. Avicequinone B sensitizes anoikis in human lung cancer cells. J Biomed Sci. 2018; 25:32. PMID: 29631569.
Article14. Miean KH, Mohamed S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J Agric Food Chem. 2001; 49:3106–3112. PMID: 11410016.
Article15. Imran M, Rauf A, Abu-Izneid T, Nadeem M, Shariati MA, Khan IA, Imran A, Orhan IE, Rizwan M, Atif M, Gondal TA, Mubarak MS. Luteolin, a flavonoid, as an anticancer agent: a review. Biomed Pharmacother. 2019; 112:108612. PMID: 30798142.
Article16. López-Lázaro M. Distribution and biological activities of the flavonoid luteolin. Mini Rev Med Chem. 2009; 9:31–59. PMID: 19149659.17. Xu T, Li D, Jiang D. Targeting cell signaling and apoptotic pathways by luteolin: cardioprotective role in rat cardiomyocytes following ischemia/reperfusion. Nutrients. 2012; 4:2008–2019. PMID: 23235403.
Article18. Birt DF, Hendrich S, Wang W. Dietary agents in cancer prevention: flavonoids and isoflavonoids. Pharmacol Ther. 2001; 90:157–177. PMID: 11578656.
Article19. Martin KR. Targeting apoptosis with dietary bioactive agents. Exp Biol Med (Maywood). 2006; 231:117–129. PMID: 16446487.
Article20. Rauf A, Imran M, Butt MS, Nadeem M, Peters DG, Mubarak MS. Resveratrol as an anti-cancer agent: a review. Crit Rev Food Sci Nutr. 2018; 58:1428–1447. PMID: 28001084.
Article21. Chen KC, Chen CY, Lin CR, Yang TY, Chen TH, Wu LC, Wu CC. Luteolin attenuates TGF-β1-induced epithelial-mesenchymal transition of lung cancer cells by interfering in the PI3K/Akt-NF-κB-Snail pathway. Life Sci. 2013; 93:924–933. PMID: 24140887.
Article22. Ma L, Peng H, Li K, Zhao R, Li L, Yu Y, Wang X, Han Z. Luteolin exerts an anticancer effect on NCI-H460 human non-small cell lung cancer cells through the induction of Sirt1-mediated apoptosis. Mol Med Rep. 2015; 12:4196–4202. PMID: 26096576.
Article23. Wang Q, Wang H, Jia Y, Ding H, Zhang L, Pan H. Luteolin reduces migration of human glioblastoma cell lines via inhibition of the p-IGF-1R/PI3K/AKT/mTOR signaling pathway. Oncol Lett. 2017; 14:3545–3551. PMID: 28927111.
Article24. Sukketsiri W, Sawangjaroen K, Tanasawet S. Anti-apoptotic effects of phyllanthin against alcohol induced liver cell death. Trop J Pharm Res. 2016; 5:981–988.25. Singkhorn S, Tantisira MH, Tanasawet S, Hutamekalin P, Wongtawatchai T, Sukketsiri W. Induction of keratinocyte migration by ECa 233 is mediated through FAK/Akt, ERK, and p38 MAPK signaling. Phytother Res. 2018; 32:1397–1403. PMID: 29532532.
Article26. Sit ST, Manser E. Rho GTPases and their role in organizing the actin cytoskeleton. J Cell Sci. 2011; 124:679–683. PMID: 21321325.
Article27. Lin Y, Shi R, Wang X, Shen HM. Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr Cancer Drug Targets. 2008; 8:634–646. PMID: 18991571.
Article28. Chen P, Zhang JY, Sha BB, Ma YE, Hu T, Ma YC, Sun H, Shi JX, Dong ZM, Li P. Luteolin inhibits cell proliferation and induces cell apoptosis via down-regulation of mitochondrial membrane potential in esophageal carcinoma cells EC1 and KYSE450. Oncotarget. 2017; 8:27471–27480. PMID: 28460467.
Article29. Cherng JM, Shieh DE, Chiang W, Chang MY, Chiang LC. Chemopreventive effects of minor dietary constituents in common foods on human cancer cells. Biosci Biotechnol Biochem. 2007; 71:1500–1504. PMID: 17587681.
Article30. Pu Y, Zhang T, Wang J, Mao Z, Duan B, Long Y, Xue F, Liu D, Liu S, Gao Z. Luteolin exerts an anticancer effect on gastric cancer cells through multiple signaling pathways and regulating miRNAs. J Cancer. 2018; 9:3669–3675. PMID: 30405835.
Article31. Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008; 83:584–594. PMID: 18452692.
Article32. Cook MT. Mechanism of metastasis suppression by luteolin in breast cancer. Breast Cancer (Dove Med Press). 2018; 10:89–100. PMID: 29928143.
Article33. Carelli S, Zadra G, Vaira V, Falleni M, Bottiglieri L, Nosotti M, Di Giulio AM, Gorio A, Bosari S. Up-regulation of focal adhesion kinase in non-small cell lung cancer. Lung Cancer. 2006; 53:263–271. PMID: 16842883.
Article34. Ji HF, Pang D, Fu SB, Jin Y, Yao L, Qi JP, Bai J. Overexpression of focal adhesion kinase correlates with increased lymph node metastasis and poor prognosis in non-small-cell lung cancer. J Cancer Res Clin Oncol. 2013; 139:429–435. PMID: 23143646.
Article35. Mazurenko NN, Kogan EA, Zborovskaya IB, Kisseljov FL. Expression of pp60c-src in human small cell and non-small cell lung carcinomas. Eur J Cancer. 1992; 28:372–377. PMID: 1375484.
Article36. Bolós V, Gasent JM, López-Tarruella S, Grande E. The dual kinase complex FAK-Src as a promising therapeutic target in cancer. Onco Targets Ther. 2010; 3:83–97. PMID: 20616959.
Article37. Carragher NO, Frame MC. Focal adhesion and actin dynamics: a place where kinases and proteases meet to promote invasion. Trends Cell Biol. 2004; 14:241–249. PMID: 15130580.
Article38. Gu MM, Gao D, Yao PA, Yu L, Yang XD, Xing CG, Zhou J, Shang ZF, Li M. p53-inducible gene 3 promotes cell migration and invasion by activating the FAK/Src pathway in lung adenocarcinoma. Cancer Sci. 2018; 109:3783–3793. PMID: 30281878.39. Schaller MD, Hildebrand JD, Shannon JD, Fox JW, Vines RR, Parsons JT. Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol Cell Biol. 1994; 14:1680–1688. PMID: 7509446.
Article40. Chen M, Chen SC, Pallen CJ. Integrin-induced tyrosine phosphorylation of protein-tyrosine phosphatase-alpha is required for cytoskeletal reorganization and cell migration. J Biol Chem. 2006; 281:11972–11980. PMID: 16507567.41. Hsia DA, Mitra SK, Hauck CR, Streblow DN, Nelson JA, Ilic D, Huang S, Li E, Nemerow GR, Leng J, Spencer KS, Cheresh DA, Schlaepfer DD. Differential regulation of cell motility and invasion by FAK. J Cell Biol. 2003; 160:753–767. PMID: 12615911.
Article42. Chikara S, Lindsey K, Borowicz P, Christofidou-Solomidou M, Reindl KM. Enterolactone alters FAK-Src signaling and suppresses migration and invasion of lung cancer cell lines. BMC Complement Altern Med. 2017; 17:30. PMID: 28068967.
Article43. Sandilands E, Frame MC. Endosomal trafficking of Src tyrosine kinase. Trends Cell Biol. 2008; 18:322–329. PMID: 18515107.
Article44. Gardel ML, Schneider IC, Aratyn-Schaus Y, Waterman CM. Mechanical integration of actin and adhesion dynamics in cell migration. Annu Rev Cell Dev Biol. 2010; 26:315–333. PMID: 19575647.
Article45. Kim LC, Song L, Haura EB. Src kinases as therapeutic targets for cancer. Nat Rev Clin Oncol. 2009; 6:587–595. PMID: 19787002.
Article46. Summy JM, Gallick GE. Src family kinases in tumor progression and metastasis. Cancer Metastasis Rev. 2003; 22:337–358. PMID: 12884910.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of Phosphatase and Tensin Homolog Deleted on Chromosome Ten (PTEN) in Lung Cancer
- Antimetastatic effect of fucoidan against non-small cell lung cancer by suppressing non-receptor tyrosine kinase and extracellular signalrelated kinase pathway
- Preferential Cytotoxic Effect of Genistein on G361 Melanoma Cells Via Inhibition of the Expression of Focal Adhesion Kinase
- Signal Transduction of Ras-like GTPase Activation in the Nervous System
- Overview of ALK and ROS1 Rearranged Lung Cancer